Here I will be focusing on a chemical surface technique called X-ray Fluorescence Spectroscopy, this method is of importance because it is widely used for chemical analysis particularly in investigation of different materials and research in forensic science this will be vital in solving criminal cases. This technique will be described on the chemical and physical levels.
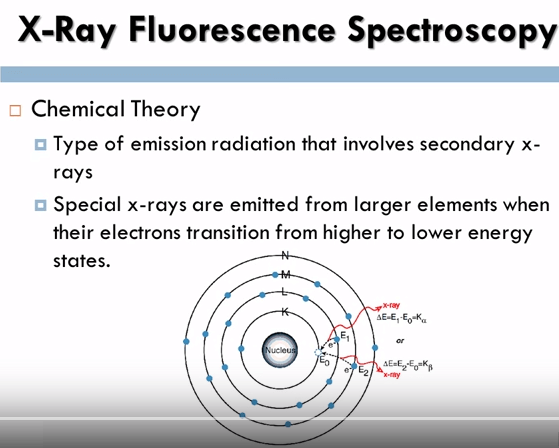
Let’s look at x-ray fluorescence spectroscopy on a chemical level first. X-ray Fluorescence Spectroscopy is a type of emission radiation that involves secondary x-rays from a source that has been stimulated by the bombarding off high energy x-rays. When a primary x-ray citation source from an x-ray tube or radioactive source strikes a sample the x-ray can either absorbed by the atom or scattered through the material.
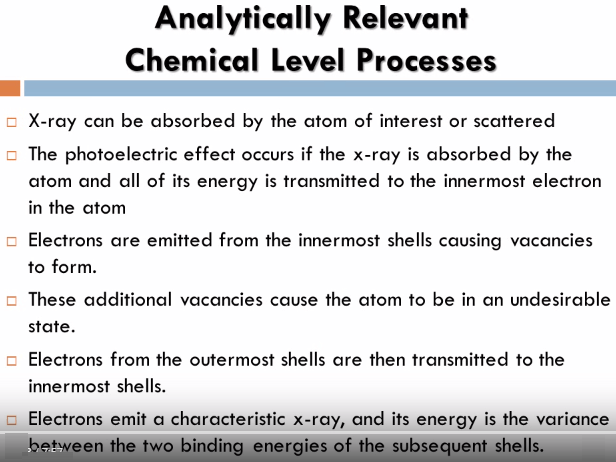
The process in which an x-ray is absorbed by the atom by transferring all of its energy to an innermost electron is called the photoelectric effect, during this process if the primary x-ray has sufficient energy electrons are ejected from the inner shells creating vacancies, these vacancies present an unstable condition for the atom, as the atom returns to its stable condition electrons from the outer shells are transferred to the inner shells and in the process give off a characteristic x-ray whose energy is a difference between the two binding energies of the corresponding shells, because each element has a unique set of energy levels, each element produces x-rays at a unique set of energies allowing one to non-destructively measure the elemental composition of a sample.
 The XRF method is widely used to measure the elemental composition of materials, since this method is fast and non-destructive to the sample it is the method of choice for field applications and industrial production for control of materials. Depending on the application XRF can be produced by using not only x-rays but also other primary excitation sources like alpha particles, protons or high energy electron beams.
The XRF method is widely used to measure the elemental composition of materials, since this method is fast and non-destructive to the sample it is the method of choice for field applications and industrial production for control of materials. Depending on the application XRF can be produced by using not only x-rays but also other primary excitation sources like alpha particles, protons or high energy electron beams.
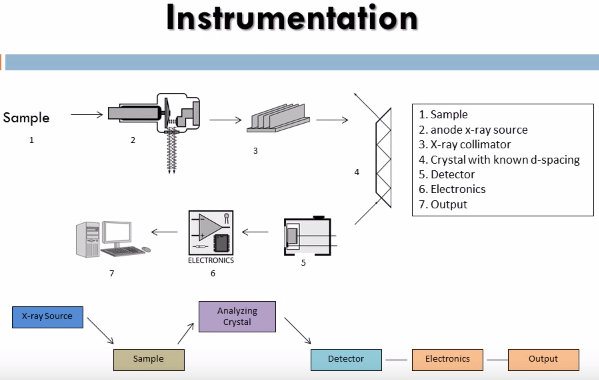 The instrument that this method utilizes is an x-ray fluorescence spectrometer it consists of an x-ray tube to produce x-rays capable of dislodging inner shell electrons of all elements of interest in the sample, these have to be high energy x-rays so: chromium, molybdenum, tungsten and silver targets are used as the anode. The x-rays that are produced in the sample are then sent through a collimator to produce a coherent beam. These are then diffracted through a crystal of known de-spacing using the Bragg equation:
The instrument that this method utilizes is an x-ray fluorescence spectrometer it consists of an x-ray tube to produce x-rays capable of dislodging inner shell electrons of all elements of interest in the sample, these have to be high energy x-rays so: chromium, molybdenum, tungsten and silver targets are used as the anode. The x-rays that are produced in the sample are then sent through a collimator to produce a coherent beam. These are then diffracted through a crystal of known de-spacing using the Bragg equation: 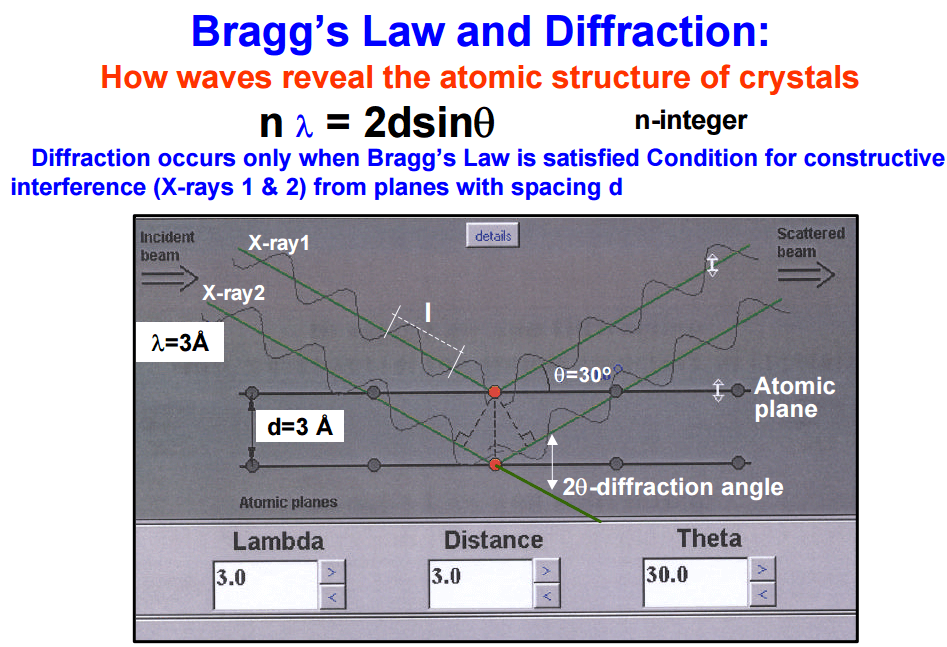
We can determine the angle that the x-rays of known wave length will be diffracted through the crystal thus the crystal and detector are set up to diffract only x-rays of the particular wave length of interest into the detector.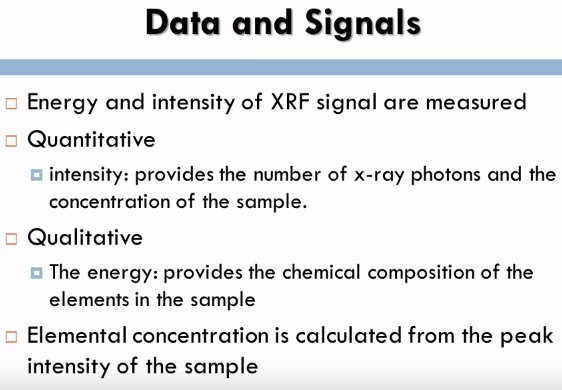
In x-ray fluorescence spectrometry both the energy and intensity of XRF signal are measured, the intensity which is on the Y axis which contains the qualitative analysis provides a number of x-ray photons and the concentration of the sample, The energy which is on the X axis which contains the qualitative analysis provides the chemical composition of the elements in the sample. The elemental concentration is calculated from the peak intensity of the sample.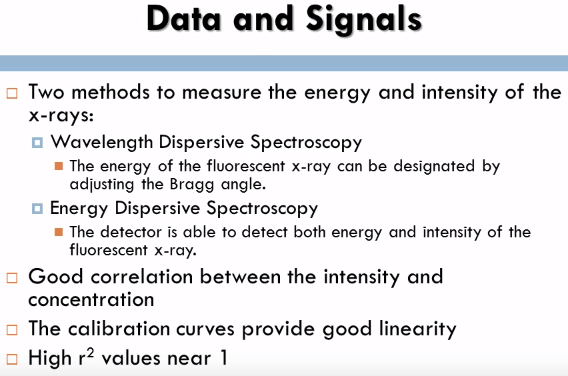
There are two methods to measure the energy and intensity of the x-rays; Wavelength Dispersive Spectroscopy and Energy Dispersive Spectroscopy. In Wavelength Dispersive Spectroscopy the energy of the fluorescent x-ray can be designated by adjusting the Bragg angle, the angle can be determined by using the Bragg condition. In Energy Dispersive Spectroscopy the detector is able to detect both energy and intensity of the fluorescent x-ray; this method is highly efficient and can detect multiple elements. This method provides a good correlation between the intensity and concentration of the elements if performed properly. The calibration curves provide good linearity with high R squared values near 1 and refining the signal and background ratio is very vital in XRF to correct the lower detection limit. 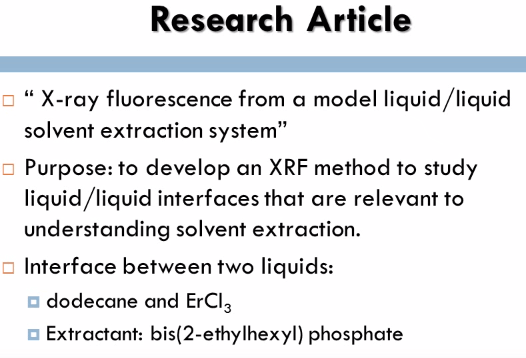 The research article that I analyzed for the x-ray fluorescence spectroscopy method was x-ray fluorescence from a model liquid/liquid solvent extraction system. For this experiment researchers were interested in the interface between two liquids; dodecane and ErCl3 because it is significant to several scientific and manufacturing applications. The process of liquid/liquid extraction, a chemical method used to isolate a specific species from a solution was utilized. X-ray fluorescence near total reflection is commonly used with the liquid gas interfaces, however in this experiment it was being utilized with the liquid/liquid interface. The purpose of this experiment was to determine the ERL shell fluorescence spectra from the liquid/liquid interface between dodecane and ErCl at different wave vector transfers and bis(2-ethylhexyl) phosphate was used as a extracting. This experiment is a continuing study to help report concerns on how to recognize and develop Lanthanide separations.
The research article that I analyzed for the x-ray fluorescence spectroscopy method was x-ray fluorescence from a model liquid/liquid solvent extraction system. For this experiment researchers were interested in the interface between two liquids; dodecane and ErCl3 because it is significant to several scientific and manufacturing applications. The process of liquid/liquid extraction, a chemical method used to isolate a specific species from a solution was utilized. X-ray fluorescence near total reflection is commonly used with the liquid gas interfaces, however in this experiment it was being utilized with the liquid/liquid interface. The purpose of this experiment was to determine the ERL shell fluorescence spectra from the liquid/liquid interface between dodecane and ErCl at different wave vector transfers and bis(2-ethylhexyl) phosphate was used as a extracting. This experiment is a continuing study to help report concerns on how to recognize and develop Lanthanide separations.
For the experiment a sample cell was utilized for the x-ray experiments, the quantitative data for the samples with varying ErCl concentrations generated a distinctive skill factor that accounted for the scattering geometry and aided in understanding the Er3+ scattering at the inner face, as a result of the data it was determined that the primary source of fluorescence from ions in the lower aqueous phase was due to secondary scattering that was made in the dodecane phase.
Overall the XRF method was of primary interest because it is relevant in several analytical processes such as forensic chemistry, drug transport, solvent extraction etc. In XRF the incident photon or electron knocks out a core electron then an excited electron relaxes by giving off fluorescence, the electrons x-ray photons have characteristic energies and thus give qualitative and quantitative data on elemental surface composition, in the experiment x-ray fluorescence from a model liquid/liquid solvent extraction system, the purpose was to develop an XRF method to study liquid/liquid interfaces that are relevant to understanding solvent extraction. In the near future XRF will be useful for studying metal ion enrichment from mixed salt solutions and it will aid in determining coordination structures of all liquid/liquid interfaces.