See many used XRF Analyzer Models and their prices. Here I list the price of a Portable XRF Analyzer (AKA handheld XRF spectrometer) depends on functionality and Brand. Used models can save you as much as 70% over new ones.
REVIEW XRF TECHNICAL SPECIFICATIONS BY BRAND: Olympus/Bruker/Niton/Oxford/Spectro
Visit our XRF Discussion Forum
Olympus offers a variety of configurations when it comes to Delta offerings. The Delta classic is SiPin technology (no light elements Ti and above) Delta Standard has a small area SDD (faster with light elements) and the Delta Pro and Premium offer a large area SDD (fast with the lowest light element detection). Here is a menu of Delta offerings and as you scroll down you will see what calibrations (modes) are available for each and what elements are available for each. Read about XRF safety and sample preparation using a pulverizer for spectrometers.
Ever wondered what an XRF can assay or what is the price of a Delta XRF Analyzer (List) by Olympus?

Look through and see what analyzer and mode would best suit your requirements and application and get a quote made up specifically for you. Ballpark pricing is around $30,000 for a Classic and $35,000 for a Delta pro and premium for new units.
Olympus offers leasing options through a 3rd party leasing company that can spread payments over 2,3,4 year term as well.
You can train for the certification process and facilitate the exam to make things easier that way. Do you know how an XRF works?
Olympus often suggests a Delta Pro with Alloy plus and precious metals addition with Karat I.D and possible a workstation for small sample analysis. You may be interested in mining plus or Geochem mode as well.

Niton by ThermoFisher are great but more money. Also see https://www.niton.com/en/niton-analyzers-products/which-xrf-analyzer-is-right-for-me
2020 Pricing examples for Niton by ThermoFisher :
- XL2 800 = $25,000
- Xl3t 800 = $39,100
- XL3t 980 = $45,800
2019 Niton
- XL3t 970 GOLDD+ = $40,000
2019 Pricing Example for Olympus XRF:
- Delta Pro = $40,000
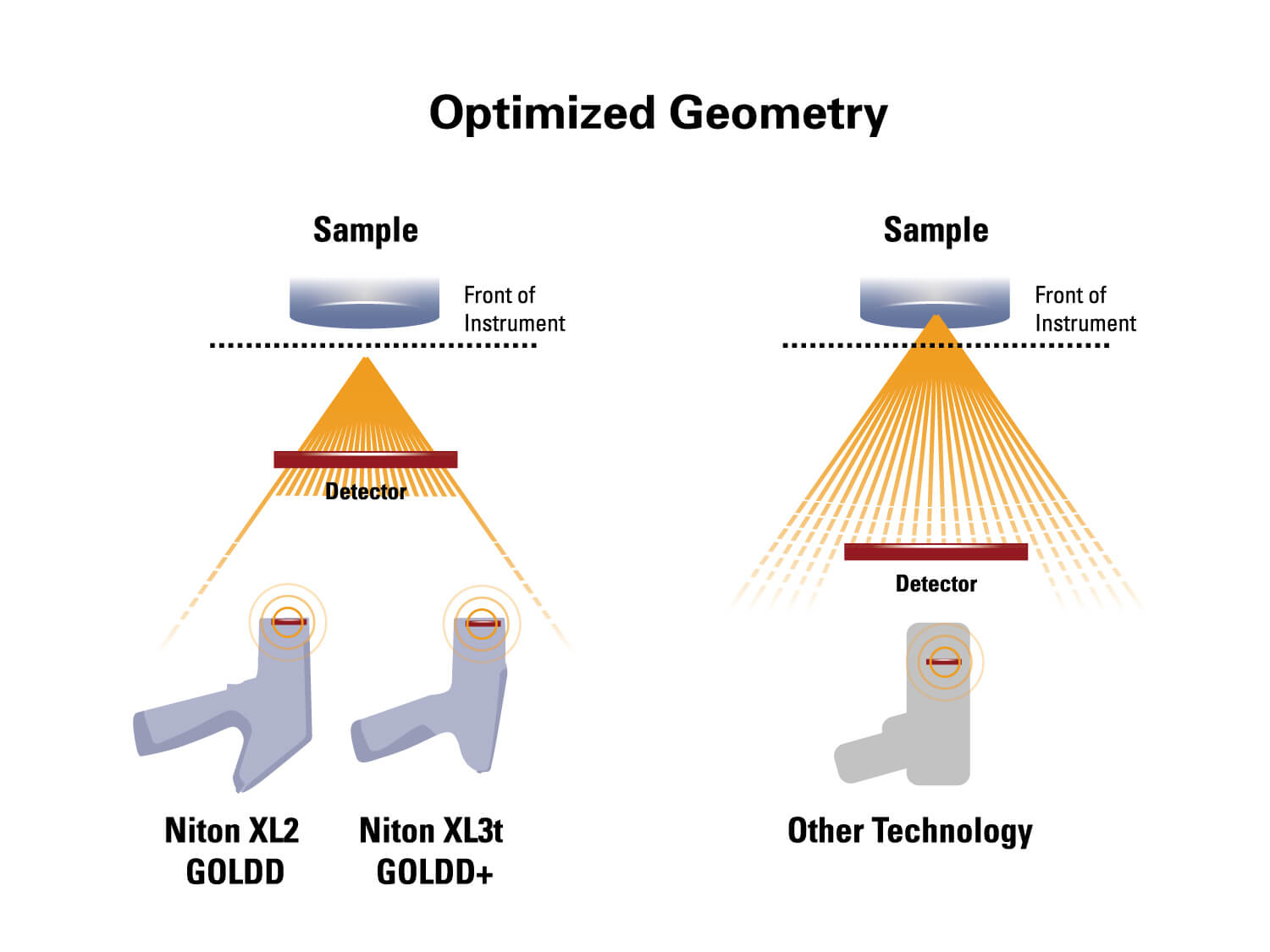
The XL3t 970 GOLDD+ Handheld XRF Analyzer with 50kV miniature x-ray tube and geometrically optimized large area drift detector (GOLDD+) and a camera for positioning small samples. Package includes the Portable Test Stand.
Standard calibrations:
- B: Electronic Metals Analysis; (Includes elemental analysis of: Ba, Sb, Sn, In, Cd, Pd, Ag, Mo, Nb, Zr, Se, Bi, Pb, Pt, Br, Au, Hg, Ta, Hf, Zn, Cu, Ni, Co, Fe, Mn, Cr, V, Ti)
- H: Plastics Analysis: (Includes elemental analysis of: Ba, Sb, Sn, Cd, Br, Se, Bi, Pb, As, Au, Hg, Zn, Cu, Ni, Fe, Cr, V, Ti, Cl)
- Ap–Consumer Paint Analysis: (Includes elemental analysis of: Pb only. For analysis of paint on consumer products only.)
- Test All TM (B, H and Ap)

Best use fire assay for gold analysis.
Mobile laboratories are important to the exploration geochemist because they offer rapid chemical analyses of geologic materials in the field. This permits modification of the sampling plan in response to significant findings, rather than following a planned sampling scheme and leaving any follow-up to a later time. The U.S. Geological Survey has long had mobile laboratories of varying degrees of mobility and complexity, ranging from truck or trailer mounted optical emission spectrographic and atomic-absorption laboratories to simple prepared colorimetric test kits carried in the field.
The portable X-ray spectrograph, Portaspec, appeared to be suitable for adding X-ray spectrographic methodology to the field techniques. It is lightweight, consists of two 25- and 8-kg pieces, is of rugged construction, and requires only an electrical power supply. With a portable generator it can be used in a field camp or vehicle.
Sampling for geochemical studies may include the collection of a variety of materials; rocks, soils, water, plant-material, stream-sediments, and panned heavy-mineral concentrates of stream sediments. All have advantages, but to be suitable for a rapid field analytical technique such as that utilizing the Portaspec, the sampling and sample preparation must be rapid and require only mobile equipment. The sampling and sample preparation should provide enough sample with the elements of interest at a concentration sufficiently high to allow the analytical technique to detect significant geochemical anomalies.
For this study, the stream sediment was selected as the sample media. Stream-sediment samples require a minimum of sample preparation time and equipment in the field. They are dried and sieved.
Stream-sediment samples, which had been previously analyzed by optical emission spectroscopy and atomic-absorption spectroscopy were used in this study so that the results could be compared. The samples came from two different environments, a probable porphyry copper deposit and a zinc-lead sulfide deposit.
The X-ray spectrograph is a Portaspec portable X-ray spectrograph (Pitchford Scientific instruments Division of Hankison Corporation) Model 2501. The power supply and readout display weighs 25 kg and the probe head weighs 8 kg. A tungsten target beryllium window X-ray tube is operated at a fixed 30 kV with a lithium fluoride (200) analyzing crystal and sealed proportional detector. The only service required is 115V, 60 Hz, 300W electrical. A recirculating system for water cooling of the X-ray tube is used.
The probe head was operated in an inverted position to present a flat surface of the sieved stream-sediment sample to the X-ray beam. An inverted sample attachment is available commercially but was not used. A cup-shaped radiation shield enclosing the sample was used to provide radiation protection for the operator.
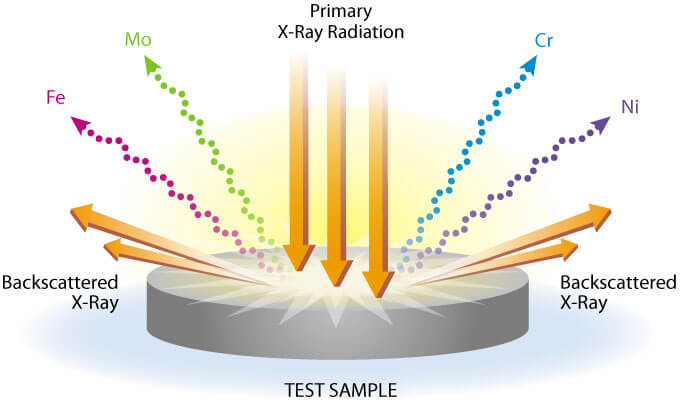
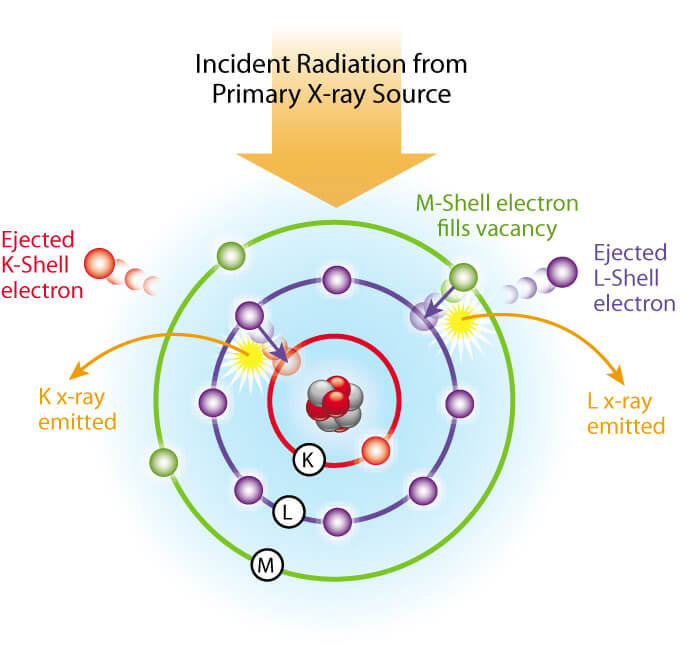
All determinations were made at 4 µm X-ray tube current for 20 seconds and read from a digital scaler. The K alpha line was used for Cu, Fe, Mn, Rb, Sr, and Zn and the L alpha line was used for Ba and Pb.
The dried stream-sediment sample is passed through a 0.58 mm opening sieve (30 mesh). A cylindrical polypropylene X-ray sample cell (23 mm diameter, 16 mm deep) is filled to near capacity and covered with 6 µm (¼ mil) mylar sheet. The inverted sample is then analyzed with the Portaspec with the above parameters.
Analytical working curves relating X-ray counts as a function of element concentration were determined for Ba, Cu, Fe, Mn, Pb, Rb, Sr, and Zn. Standards for Fe, Mn, Rb, and Sr were prepared in a matrix containing Si, Al, Ca, Mg, Na, and K approximating the composition of the stream-sediment samples. Standards for Ba, Cu, Pb, and Zn were prepared by adding oxides of Cu, Pb, and Zn and BaCO3 to a clean stream-sediment sample of granitic origin containing no detectable Pb and Zn and less than 5 ppm Cu determined by emission spectroscopy. The lower useful range of the analytical working curve for all elements was 100 to 200 ppm and was established for concentrations as great as 10 percent for Fe; 5 percent for Ba, Cu, Pb, and Zn; 1 percent for Mn and Sr; and 0.5 percent for Rb.
The precision for the determination of approximately 2,000 ppm Cu, Mn, Rb, Sr, and Zn; 5,000 ppm Ba and Pb; and 2.3 percent Fe as determined from 20 replicate analyses of a granitic stream-sediment sample is shown in Table 1.
The effect of varying amounts of the rock-forming elements iron, calcium, and magnesium on the determination of approximately 2,000 ppm Cu, Mn, Rb, Sr, and Zn; and 5,000 ppm Ba and Pb was studied by adding 1, 2, and 5 percent Fe and Ca and 0.5, 1, and 2 percent Mg to a granitic stream-sediment sample. This sample had been determined to contain approximately 3 percent Fe, 2 percent Ca, and 1.5 percent Mg by emission spectroscopy prior to the above additions. The general decrease in apparent concentrations of Ba, Cu, Mn, Pb, Rb, Sr, and Zn produced by the addition of Fe, Ca, and Mg is shown in Figure 1. The suppression produced by Fe is greater than Ca, which is greater than Mg. The effect is generally greatest on Pb and Ba and least on Cu and Zn. Variations in iron, calcium, and magnesium concentrations encountered in stream-sediments samples will have an effect on the determination of from 100 to several thousand ppm concentrations of the elements studied.
Analysis of ore-grade material is subject to more serious matrix effects. Table 2 shows the analysis of six geochemical reference samples (Allcott and Lakin, 1978) for copper, lead, and zinc. These six samples exhibit a wide range of percentage composition (Fe 2-25, Ca 0.1-14, Mg 0.2-1.5, and Mn 0.015-0.5) as well as having ppm concentrations as great as As, 5900; Cu, 6500; and W, 3800. Table 2 shows the departure of counts to concentration from linear or curvelinear relationships due to absorption or interferences. The high concentrations of iron in samples GXR1 and GXR3 absorb the radiation from all three elements. An interference on the lead L-alpha line of GXR3 is due to the K alpha line from 4,000 ppm arsenic. The high copper concentration (6,500 ppm) in GXR4 interferes with both the zinc K alpha line and background measured at 40.5° 2θ. Attempts to correct element counts with background taken at 40.5° and 46.5° 2θ or using the tungsten X-ray tube target radiation as an internal standard were not successful.
The effect of high concentrations of copper on the determination of zinc noted with the samples in Table 2 was examined further when copper was added to a granitic stream-sediment sample (Table 3).
Stream-sediment samples from Sonora and Alaska were analyzed with the portable X-ray spectrograph by emission spectroscopy, and in some cases, by atomic-absorption spectroscopy. A comparison of the results for ten samples from Sonora selected as having a wide range in concentrations for copper, lead, and zinc is shown in Table 4. The atomic-absorption determinations were made following two different dissolutions, a partial nitric acid dissolution and a more complete hydrochloric-hydrofluoric acid dissolution. The different methods show good correlation despite significant bias.
In addition to the ten samples compared in Table 4, a set of 48 samples from Sonora and 74 samples from Alaska were compared. The high correlation of X-ray analyses with emission and atomic absorption analyses for all three sets of data is shown in Table 5. For calculation of correlation coefficients, an “N” was assigned a value of 0.5 of the detection limit and an “L”, 0. 7. Where no correlation is shown, the atomic absorption analysis was not made.
The usefulness of the portable X-ray spectrograph in geochemical exploration is best evidenced by the results produced. Does it locate anomalous mineralized areas? Previously collected stream sediment-samples from Koiyaktot Mountain, located in the Howard Pass 1° x 3° quadrangle of the western Brooks Range were analyzed with the Portaspec. The distribution of zinc is shown in Figure 2. A map of the previously determined mineral potential of this area is shown in Figure 3. This map summarizes the analyses by emission and atomic-absorption spectroscopy for silver, cadmium, lead, and zinc in a variety of sampling media (stream-sediment samples, nonmagnetic fraction of heavy-mineral concentrates, oxalic acid leach of iron- manganese oxide pebble coatings, and oxalic acid leach of veined stream rocks).
Rapid analyses in the field can be performed on easily obtained stream-sediment samples. The precision is approximately 1 to 3 percent relative standard deviation. Limitations are: (1) detection limits are about 100 ppm for most elements; (2) analyses may be significantly affected by high concentrations of other elements; and (3) a significant bias may exist with other methods of analysis. A geochemical anomaly similar to that at Koiyaktot can be found in the field using X-ray stream-sediment data.
The major usefulness of the instrument would be in providing timely information in the field on which decisions regarding the conduct of field operations could be based. The method would supplement other methods of analysis and sample media.