Table of Contents
Nearly all alloy steels containing tungsten are made in the electric furnace where tungsten is introduced to the steel’s metallurgy in the form of ferro-tungsten. Ferro-tungsten, melts between 3500 and 3700 degrees F., while tungsten powder melts between 6000 and 6200 degrees F. Neither will actually melt in a steel-making furnace, but the ferro-alloy because of its lower fusion temperature is more easily dissolved in the molten steel. It has a lower gravity than tungsten powder and settles slower through the melt to give a more uniform distribution.
Tungsten is definitely a metal of the twentieth century. Prior to 1900 it had almost no commercial use although it was an important but unsuspected part of the legendary Damascus steel. Tungsten was first identified in 1847 in Bohemia and the metal was isolated in Spain in 1783. Tungsten alloy steel was later discovered to be vastly superior for cutting metal at high speed and created a sensation at the Paris exposition in 1900. Cutting tools made of it could be used at high temperatures that would ruin any carbon steel.
What is Tungsten Used For
Tungsten can be heated to higher temperatures without softening than any other substance except carbon because its melting point—about 3410 degrees C (6182 degrees F)—is the highest of all metals. Tungsten is indispensable as filament wire in incandescent lamps but its consumption for this purpose is small, being only 1 to 2 percent of the annual total production. One pound of tungsten drawn into a wire 0.0022 inches in diameter provides 6.2 miles of wire or filament for 14,400 lamp bulbs of 60 watt size.
Tungsten has the highest tensile strength of any known metal—up to 600,000 lbs. per square inch. At ordinary temperatures it is not affected by acids including Aqua Regia and is soluble only in a mixture of hydrofluoric and nitric acids.
Some of the tungsten reduced to metallic form is used in making cemented carbides. High purity tungsten powder is combined with carbon at high temperature in an inert atmosphere. The resulting carbide is crushed and mixed with cobalt powder which acts as a cementing agent when the material is sintered. The result is a cemented tungsten carbide, the hardest known artificial substance. Cemented carbides are used in making rock drill bits, dies, and many types of cutting tools such as single-point tools, forming tools, milling cutters, etc.
The growth in the use of tungsten is very well illustrated in the following diagram prepared by the Wah Chang Trading Corporation, one of the principle processors of tungsten ores and its products. See Diagram (A)
Tungsten Minerals
The principle tungsten minerals and their distinguishing characteristics are given in the following tabulation:
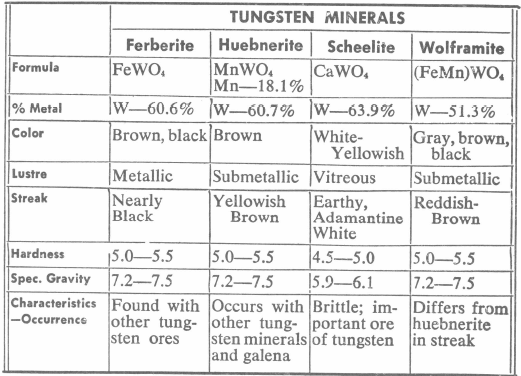
The grading, analysis, and marketing of tungsten ore is based on the tungsten trioxide (WO3) content and not on the metal content. Scheelite, the principle ore, for example, when pure, assays 80.6% WO3, and wolframite, 67.3 %WO3.
Deposits are usually placers or fissure veins; less commonly it occurs in pegmatites and in contact metamorphic zones. Gangue minerals are quartz, fluorite, cassiterite, tourmaline, garnet, mica and sulfides. Country rock is commonly granite, less frequently quartzite, limestone and metamorphic rocks.
Tungsten Ore Geology
Scheelite, the principle ore of tungsten, has a specific gravity of approximately 6.0 (slightly heavier than iron sulfides) and is soft enough to be scratched with a knife. It is usually buff or pale yellowish-brown and has a lustre that closely resembles some of the common non-metallic minerals such a calcite, feldspar, and quartz. Calcite is slightly softer and effervesces with hydrochloric acid while quartz and feldspar are lighter and harder. Scheelite may also be pure white and massive resembling barite which is also a heavy mineral having a specific gravity of 4.3-4.6.
A good geology field test is to place a small drop of hydrochloric acid on the suspected mineral and saw it for about 15 seconds with the clean blade of a knife. If it is scheelite, the cut turns blue-grey, and if the knife blade is wiped on white paper, the blue stain is well demonstrated. The blue cut darkens in a few minutes and in an hour disappears.
The best distinguishing test for scheelite is by means of a mineralight which gives off short wave ultraviolet light. Scheelite fluoresces a brilliant blue- white and is readily distinguished by this means. Occasionally other minerals may be encountered that give almost exactly the same fluorescence as scheelite so the presence of tungsten should always be verified by chemical test. The mineralight, however, is an important tool in tungsten prospecting, grading, and milling. It is extensively used for this purpose and has greatly aided in the discovery and development of many new and important properties in the past few years.
Harz Type Jig Is used In some Tungsten Mills to recover coarse minerals.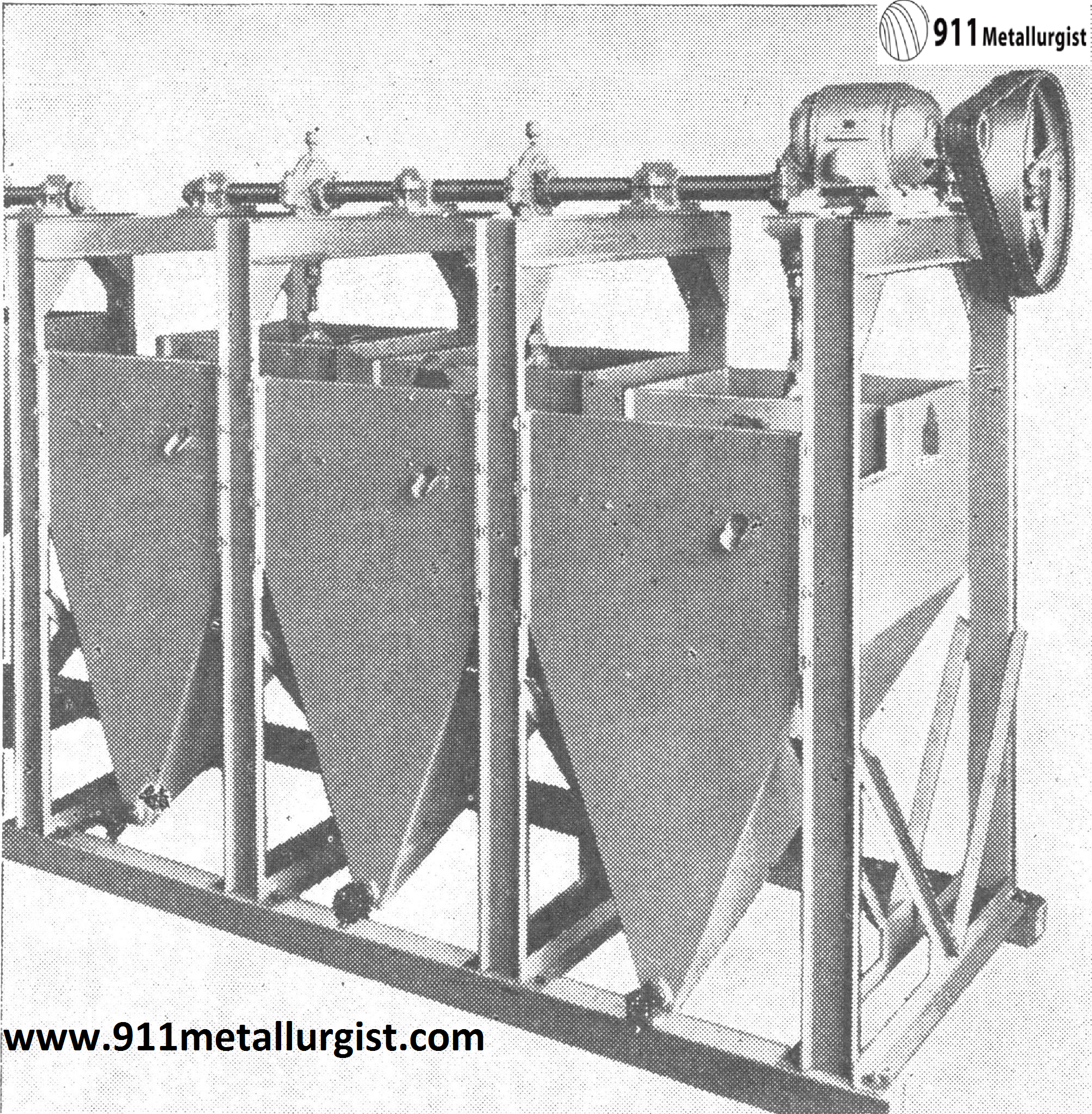
Since loss of values in fine sizes is a problem in tungsten concentration the use of crushing rolls to avoid over-crushing is normally practiced.
Wolframite and ferberite, the iron tungstates, have a dark red-brown to black metallic lustre and a dark red-brown streak. Wolframite is very difficult to identify positively in the field as it closely resembles other heavy, black minerals, such as specular hematite, magnetite, ilmenite, etc. It is non-magnetic, or is only slightly magnetic if there is admixed magnetite. The pure mineral readily fuses under the blowpipe to a bead which is magnetic. It does not fluoresce under the mineralight. Panning is a good method of detecting both scheelite and wolframite as they both appear at the tail of the pan.
A fairly simple chemical test for any tungsten ore is to grind to a fine powder and boil with concentrated hydrochloric acid for 15 minutes. If tungsten is present, the solution turns cloudy yellow and will turn blue if a piece of aluminum, zinc, solder, or tin is added. The yellow residue is soluble in ammonia.
Tungsten Ore Specifications
All tungsten ores require concentration to produce a marketable product. Generally the WO3 content of tungsten ores is less than one percent and ores with up to 3% are considered high grade. Following are specifications for tungsten concentrates with limits on impurity minerals:
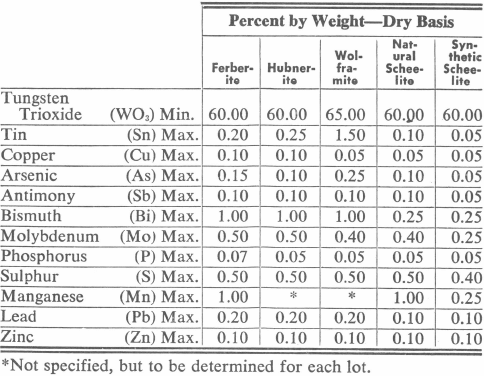
Lower grade products, such as flotation concentrates, are often marketed for further treatment by chemical means. In this case, the impurities are separated or removed chemically and the dissolved tungsten precipitated out to form artificial scheelite, CaWO4, which then is marketable in accordance with the specifications for synthetic scheelite with the further specification that not over 10% shall pass a 35 mesh Tyler standard screen. This usually means sintering or fusion to minimize the percentage of fines.
Treatment of Tungsten Ores
Tungsten minerals are generally very friable or brittle and therefore every precaution must be taken in grinding to minimize sliming. “Recover your mineral as soon as free,” is very important for all tungsten ores since tungsten minerals are not only brittle but heavy and will definitely be overground in conventional closed circuit grinding systems. It is for this reason stage crushing, screening, and gravity concentration are always incorporated in the concentration of tungsten minerals.
Methods generally used for treatment of tungsten ores or products may be tabulated as follows:
Gravity Concentration
Tungsten minerals because of their high specific gravity are very amenable to gravity concentration and this is by far, the most important method employed in the industry. Coarse and fine jigging and tabling are very common in many of the operating plant flowsheets. For tabling, careful and close hydraulic classification is usually essential, plus the use of both sand and slime decks and regrinding and retreatment of table middlings.
The main difficulty with all the gravity systems is loss of tungsten in fine sizes. This has been minimized to a large degree by careful stage crushing and screening and by use of the Mineral Jig in the grinding circuit. An important amount of fine tungsten escapes the slime tables. Rag plants are often used to save some tungsten from the slimes but they require considerable operating attention and clean-up to give extra recovery.
 Sampling is an important part of tungsten mill control. This Snyder type sampler is used on tungsten feed after crushing.
Sampling is an important part of tungsten mill control. This Snyder type sampler is used on tungsten feed after crushing.
Mineral Jig in Tungsten Circuits
One of the largest and most important uses of the Mineral Jig has been in tungsten gravity circuits. This jig is a highly efficient mineral selector which functions best on an unclassified feed and is capable of making a high grade concentrate. Ball or rod mill discharges and screen undersize products are effectively treated without further preparation or overdilution.
In one of the Colorado tungsten operations it was found very effective to use the following flowsheet incorporating the Mineral Jig which replaced coarse jigs and greatly simplified the plant flowsheet and improved recovery of tungsten which was present primarily as wolframite.
In the flowsheet below the Mineral Jig treated minus ¼” feed and was equipped with center draw-offs to remove coarse tungsten concentrates in addition to the fine hutch concentrates passing through the bedding. Over 60% of the total mill production of tungsten concentrates was recovered in a marketable product by the Mineral Jig in this circuit and as a result, overgrinding and tailing losses were far below previous mill practice.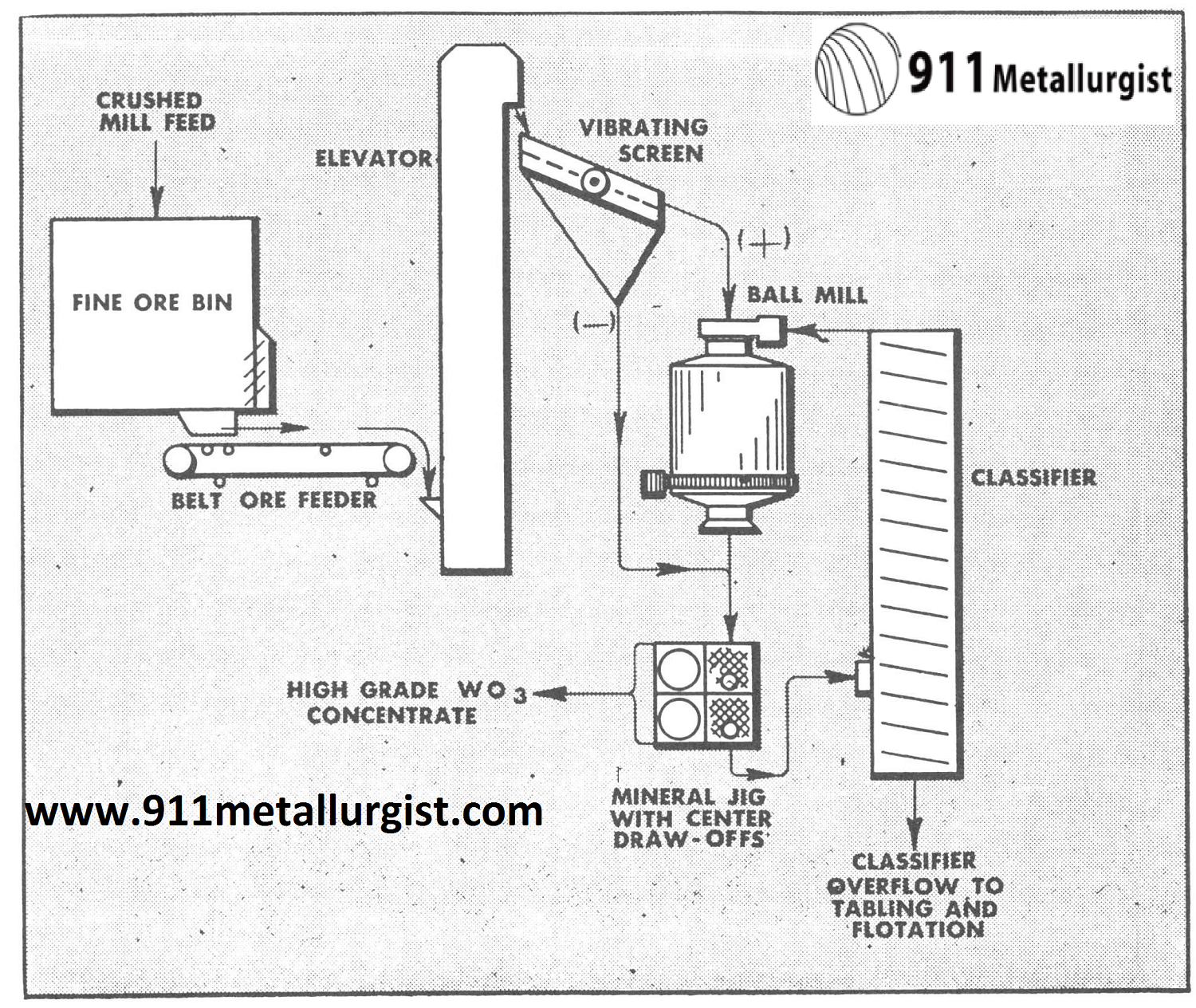
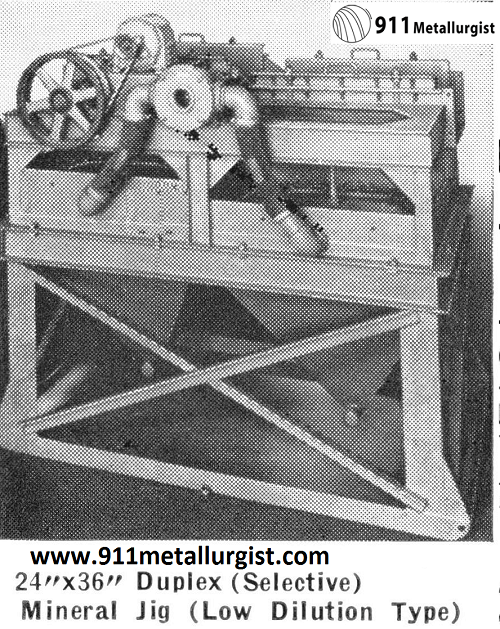
16″ x 24″ Duplex Mineral Jig showing operation of center draw-off. A side draw-off, to remove coarse mineral particles, is furnished on the Harz Type Jig.
See Diagram (C)
Even with ores containing an appreciable amount of heavy sulfide it is advisable to use the Mineral Jig in the grinding circuit for tungsten recovery. In such cases, however, the resulting concentrates will require further treatment to remove the sulfides from the mixed concentrate and upgrade the tungsten to meet market specifications. This is particularly important on ores which are erratic and spotty in their tungsten content for the removal of these “hot spots” from the ore early in the treatment process will ensure uniform operation and maximum recovery in the rest of the milling circuit.
Selective Mineral Jig is standard in tungsten concentration. Its ability to take an unclassified feed such as direct from the ball or rod mill and recover both coarse and fine minerals has solved many difficult milling problems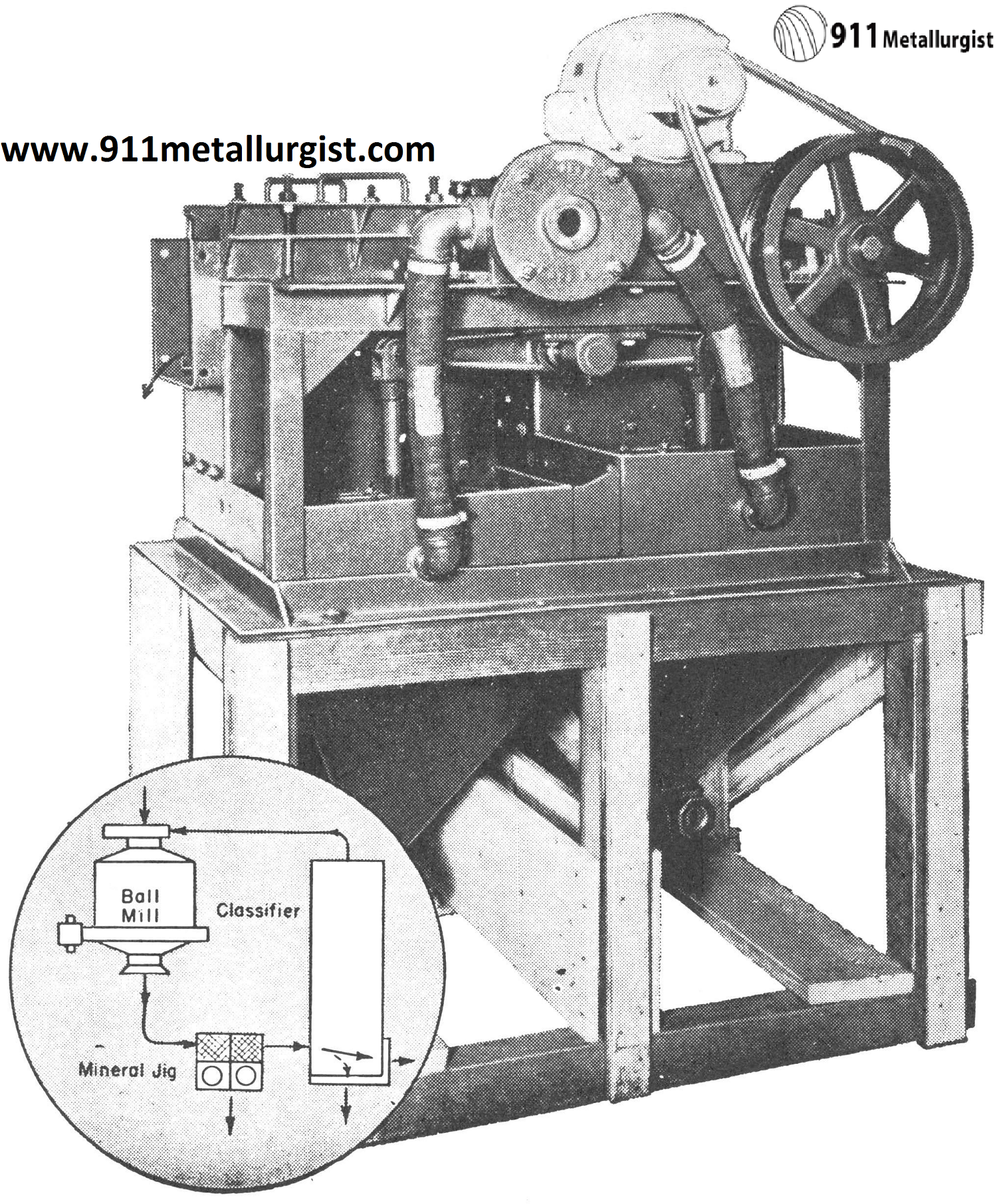
Laboratory Tests
In laboratory batch testing, it is difficult, if not impossible, to duplicate closed circuit grinding and classification conditions as occur in mill operation. It is usually possible in the laboratory to show good recovery by conventional tabling methods. Rarely can laboratory results be duplicated in actual milling practice where the tungsten minerals are often overground excessively before overflowing the classifier and reaching the tables.
For example, one of the large molybdenum operations containing a trace amount of tungsten in its ore grinds in closed circuit with a Classifier overflowing at -20 mesh and 50% solids. The tungsten mineral, wolframite, saved in subsequent treatment through a by-products plant by gravity is all -100 mesh. This clearly illustrates that heavier tungsten will not overflow the classifier until it is reduced to sizes much finer than the gangue. The Mineral Jig offers a means of doing this without upsetting the density requirements of the grinding circuit.
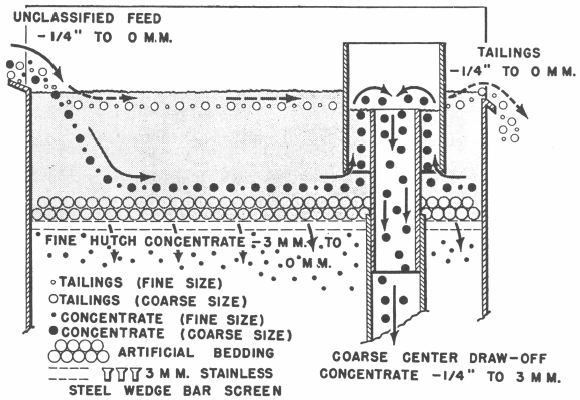
Diagramatic sketch of center draw-off operation. Note that coarse particles too large to pass through bedding screen are drawn off by passing over the top of the draw-off pipe.
Laboratory test results on a high grade wolframite ore containing a small amount of scheelite are given below to show the high grade and recovery obtained by the Mineral Jig. In this case the ore was crushed to -¼” and fed to the jig which was provided with a center draw-off to remove the coarse concentrate accumulating in the jig bedding:

Additional recovery was made by grinding the jig tailing in a rod mill in closed circuit with a 28 mesh screen and tabling the -28 mesh product.
On an average scheelite ore with a siliceous gangue containing some garnet but no sulphides, the Mineral Jig showed the following results when the ore was crushed to minus 10 mesh.

Examination of the Mineral Jig scheelite concentrate showed mineral particles from 10 mesh down to 200 mesh and a small amount of garnet. This impurity mineral was also high in gravity and further upgrading of the concentrate was accomplished by high intensity magnetic separation in order to meet marketing requirements of at least 60% WO3.
Vibrating Screens in operation in a tungsten mill show how over-crushing is reduced by removing fines from crusher feed.
Buckman Concentrator
For the recovery of fine size range of minerals by gravity, the Buckman Concentrator was developed and is being used very successfully in many of the tungsten plants. One of the tungsten operations in Bolivia was losing an appreciable amount of wolframite in their -270 mesh slimes. Treating this fraction over the Buckman Concentrator recovered 49.6% of the tungsten formerly lost in the slime table tailings. Of the tungsten slimes fed to the Buckman, 67.7% was plus 9 microns so that recovery of about 70% on the minus 270 mesh plus 1500 mesh (about 10 microns) was indicated.
 “Sub-A” Flotation Machines floating tungsten in a large Colorado concentrator.
“Sub-A” Flotation Machines floating tungsten in a large Colorado concentrator.
The development of the tilting concentrator has been going on for many years. It was used in the early 1900’s in the Boulder, Colorado area, but it has been only in recent years that it was made a success mechanically. Extensive tests were undertaken by the Consolidated Mining and Smelting Company who developed a special deck covering a waffle type rubber matting which is now being used so successfully on the Buckman Concentrator for both tin and tungsten. In some cases canvas deck covering has given very good performance on tungsten.
Accurate reagent control is made possible with Wet Reagent Feeders. Fatty acids or fatty acid soaps such as oleic acid or sodium oleate with alkaline dispersing agents are common reagents used for scheelite flotation.
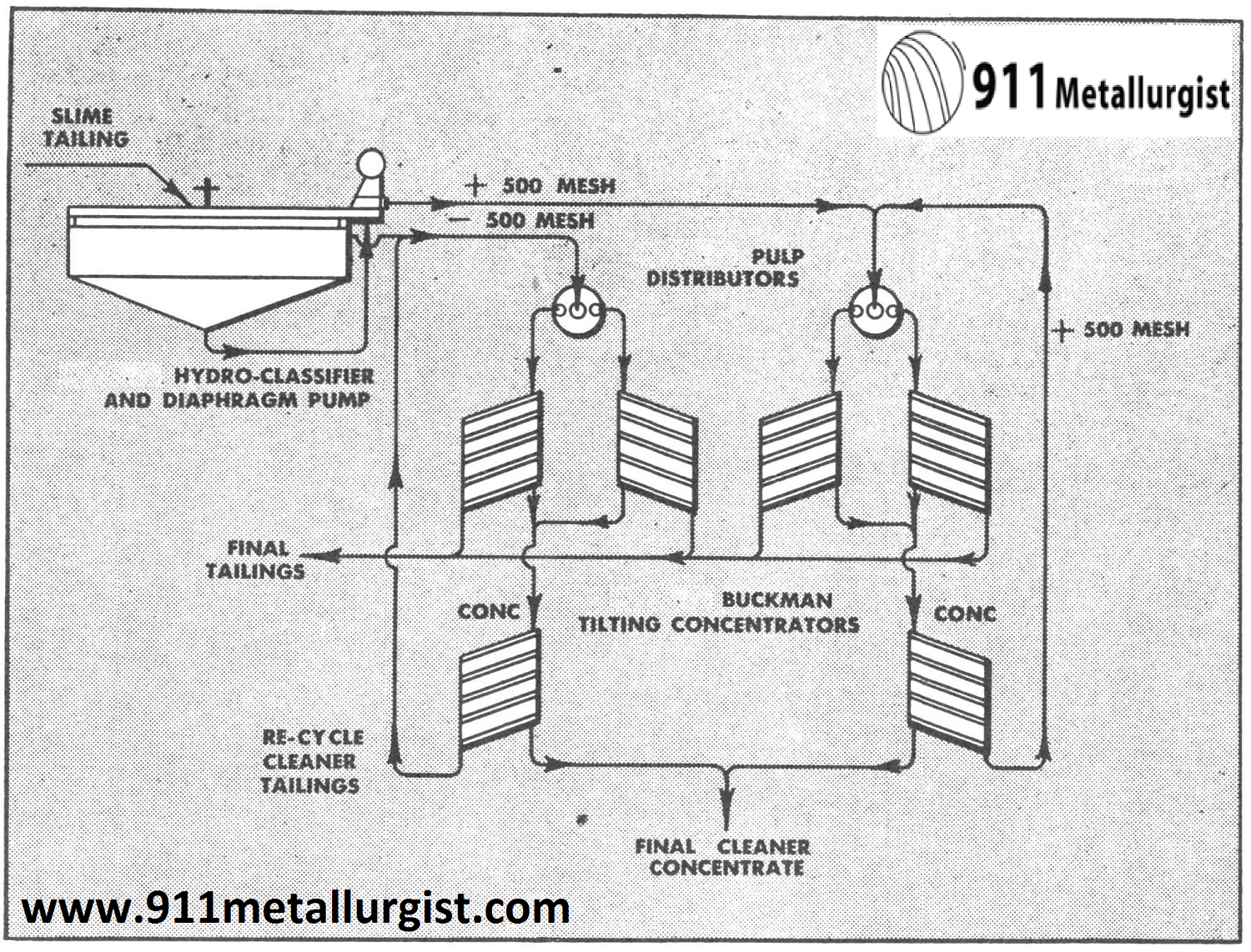
Flowsheet showing Buckman Concentrators
Generally the Buckman Tilting Concentrator is suitable for recovering mineral in the -150 to 1000 mesh range. Coarser material cuts down the efficiency and capacity of the unit. In recent practice, careful sizing of the fines has improved operating efficiency of the tilting concentrator. The above flowsheet is being used in one of the plants with considerable success when hydraulically sizing the feed at 500 mesh and treating the two fractions separately. This will vary according to the physical characteristics of the specific mineral being treated.
The cross-wash from concentrating tables in tungsten plants generally contain the bulk of the liberated tungsten lost in the tailings. This cross-wash product after thickening from about 7% solids to 15-17% solids is ideal for retreatment on the Buckman Tilting Concentrator and can readily be graded up to about 20% WO3.
Flotation of Tungsten Ores
Flotation has a very important application in treating the more complex tungsten ores, particularly those containing sulphides. Many of the tungsten ores contain pyrite, arsenopyrite, molybdenum, bismuth, copper, lead, and zinc minerals all of which would contaminate gravity tungsten concentrates. It is, therefore, very important to remove these sulfides from the resulting gravity concentrates or from the ore prior to gravity treatment. This, however, precludes initial coarse gravity treatment and necessitates grinding the ore to flotation size for effective removal of the sulphides. This reduces the size of the tungsten mineral so it becomes increasingly important to employ up-to-date and the best type equipment for gravity recovery of the tungsten fines after the sulfides have been removed by flotation.
In addition to removing sulfides, flotation is becoming well established in the recovery of the slimed tungsten minerals. In some cases it has been found advantageous to simplify the flowsheet and go to all flotation treatment for tungsten recovery and resort to chemical treatment for final up-grading of the concentrate and removal of impurities in order to meet marketing specifications.
In many tungsten gravity operations containing a small amount of sulphides, the gravity concentrates must be treated for sulphide removal. This can be accomplished by roasting, magnetic treatment, and re-tabling for removal, of iron oxides, but it is preferable to treat the concentrates through “Sub-A” flotation for sulphide removal either on a continuous or batch basis. Where coarse jig concentrates are produced containing sulphides, it is necessary to crush or rod mill down to flotation size in closed circuit with a vibrating screen. Batch treatment of tungsten concentrates in a Unit Cell has been very effective for sulphide removal. The cell in this case, is arranged for continuous circulation of the pulp within the machine and at the same time, a sulphide froth product can be removed which is very low in tungsten. The unfloated tailings from the machine are dewatered, dried, and bagged as high grade tungsten concentrates or given heat and chemical treatment in case other non-floatable impurities such as phosphorus may be present.
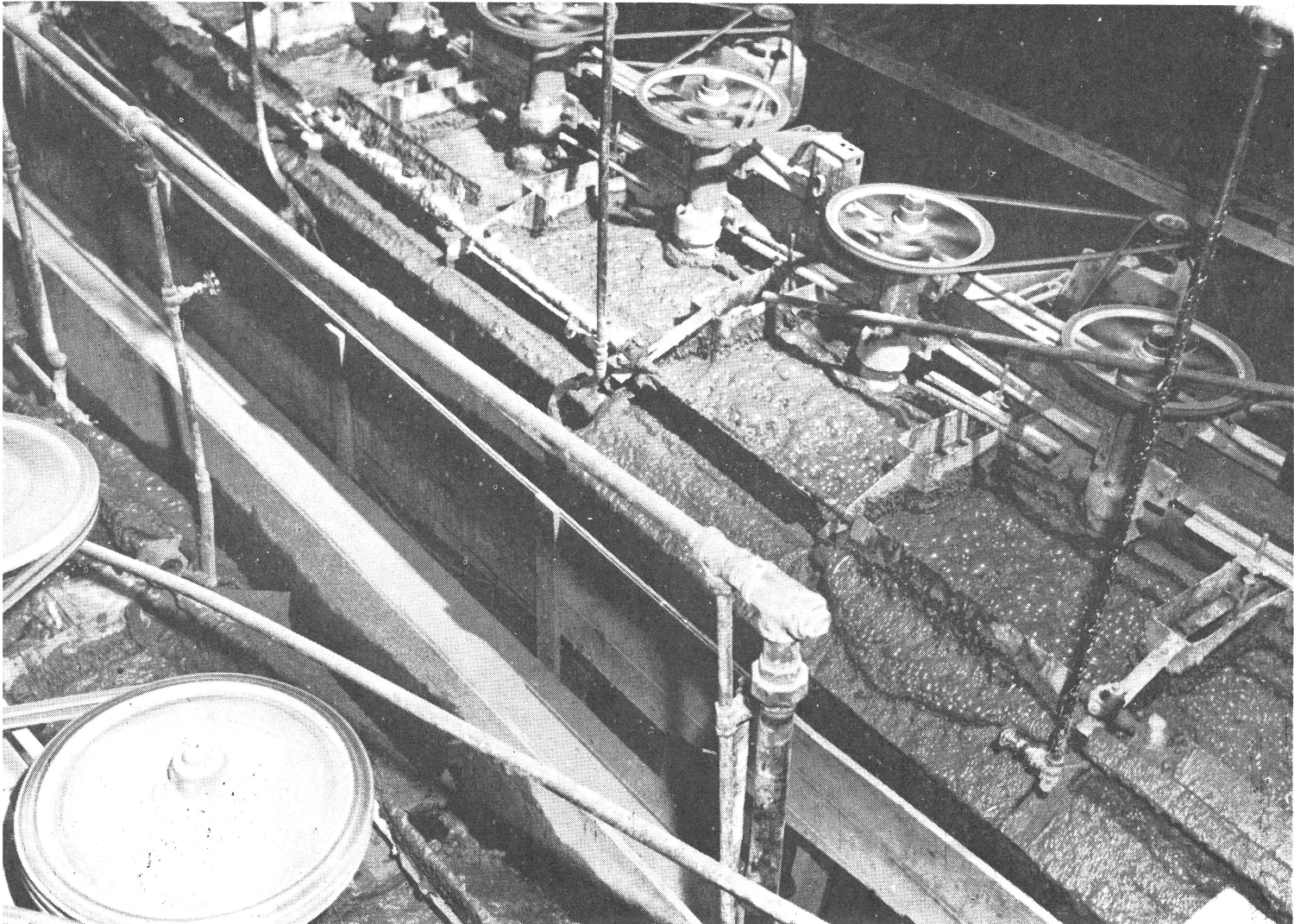 Note the soapy appearance of froth which Is typical of tungsten flotation. These No. 18 (28 x 28) “Sub-A” Cells are treating a gravity plant slime tailing which has been thickened to the proper flotation density.
Note the soapy appearance of froth which Is typical of tungsten flotation. These No. 18 (28 x 28) “Sub-A” Cells are treating a gravity plant slime tailing which has been thickened to the proper flotation density.
Scheelite is the easiest of the tungsten minerals to recover by flotation. Fatty acids or fatty acid soaps, such as oleic acid or sodium oleate with an alkaline dispersing agent such as sodium silicate, soda ash or caustic soda, are the common reagents which are used for scheelite. Orso, a neutral soap powder of vegetable origin has also been used very effectively as a flotation promoter and collector for scheelite. Emcol X-25 is sometimes very helpful.
Soft water is desirable and aids in improving selectivity and keeping reagent costs at a minimum. Apatite, if present, will float and contaminate the scheelite concentrate with phosphorus. This impurity can be effectively depressed by use of either organic or inorganic acids such as formic, hydrochloric, sulphuric, or sulphurous. Only a small amount of acid to make a neutral or slightly acid pulp is necessary, and it may be added to the rougher or cleaners. Calcite has been effectively depressed with quebracho, a crude tannic acid product.
Wolframite, hubnerite, and ferberite may all be floated but not so easily as scheelite. The same general reagents are used. Acidified dichromates are quite effective in giving clean separations on ferberite with depression of fluorite and apatite. Mixed concentrates of scheelite and wolframite can readily be separated by flotation due to the greater floatability of scheelite.
Tungsten flotation concentrates, if medium or low grade, are often thoroughly dispersed with caustic starch and tabled to scalp out a high grade marketable product. The resulting table tailing containing the balance of the tungsten may then be chemically treated to extract the tungsten which is precipitated from the liquor as synthetic scheelite, CaWO4.
Tungsten slime losses in many gravity circuits amount to as much as 20 to 30%, so with present prices and demand for this important alloying metal it is increasingly important to consider and adopt the use of flotation.
Magnetic Separation
Garnet and epidote are usually present in the contact metamorphic deposits along with tungsten. These minerals contaminate tungsten gravity concentrates and are usually removed from the dried concentrates by high intensity magnetic separation. Magnetite and ilmenite may also be present and are removed in a similar manner. Cross belt type high intensity magnetic separators have proven very satisfactory in tungsten treatment.
Small amounts of pyrite, if present in tungsten concentrate, may be rendered magnetic by superficial roasting. It is only necessary to alter the surface of the pyrite in order to make it magnetic. This is desirable on coarse concentrates where it may not be desirable to grind and float in order to remove the pyrite particles, assuming they are free from the tungsten minerals.
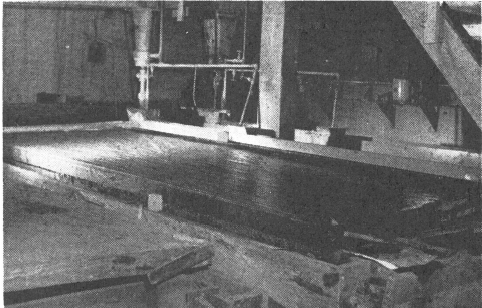
Wilfley Tables have been standard equipment in tungsten mills for many, many years. Tables are used to recover fine tungsten, to separate flotation concentrates into various products and to produce middling products that are returned for retreatment.
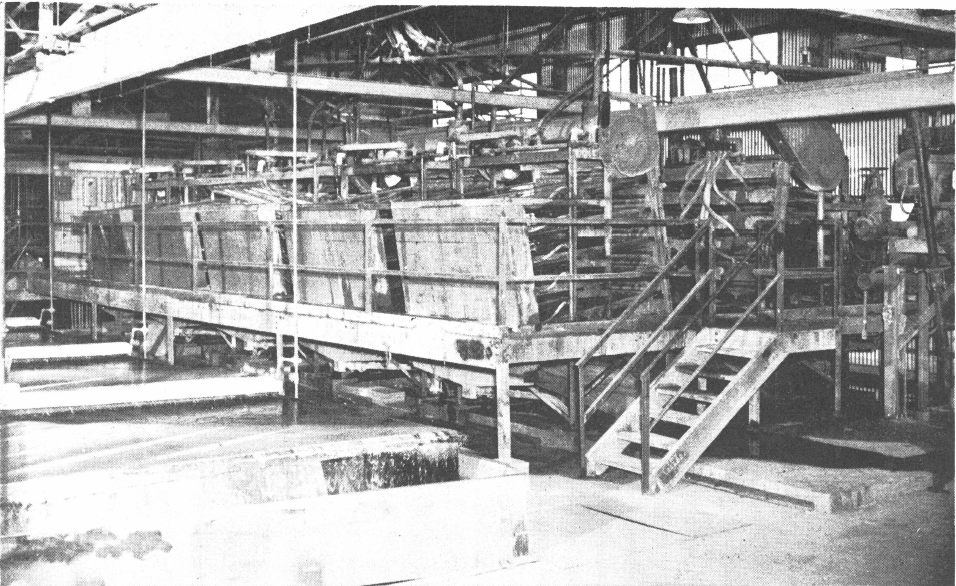
Normally several tables are used due to the sized feed required for efficient table operation. These 3 Wilfley Tables are part of the gravity concentration section in a Colorado tungsten mill.
Buckman Tilting Concentrators are very effective in recovering the extremely fine values in tungsten slimes. The above bank of concentrators in the southeastern part of the U.S. are used on tungsten slime tailings.
Tungsten Process & Circuit Flowsheets
It is very difficult to select a tungsten flowsheet that is typical of any one district as there are wide variations in the ores and methods of treatment. To ensure proper selection for a new plant it is important to obtain a representative sample and perform a complete laboratory investigation by gravity and flotation methods. This will establish at what mesh the tungsten minerals are free and to what extent coarse gravity methods can be used to recover the mineral before it has a chance to become slimed. Impurities are also ascertained and will influence to what degree flotation, magnetic separation and chemical methods will have to be employed.
Some of the more typical flowsheets are being presented in this paper to show the variations possible and to what extent the major tungsten producers have gone in their efforts to obtain the maximum economic return of this critical metal from their ores.
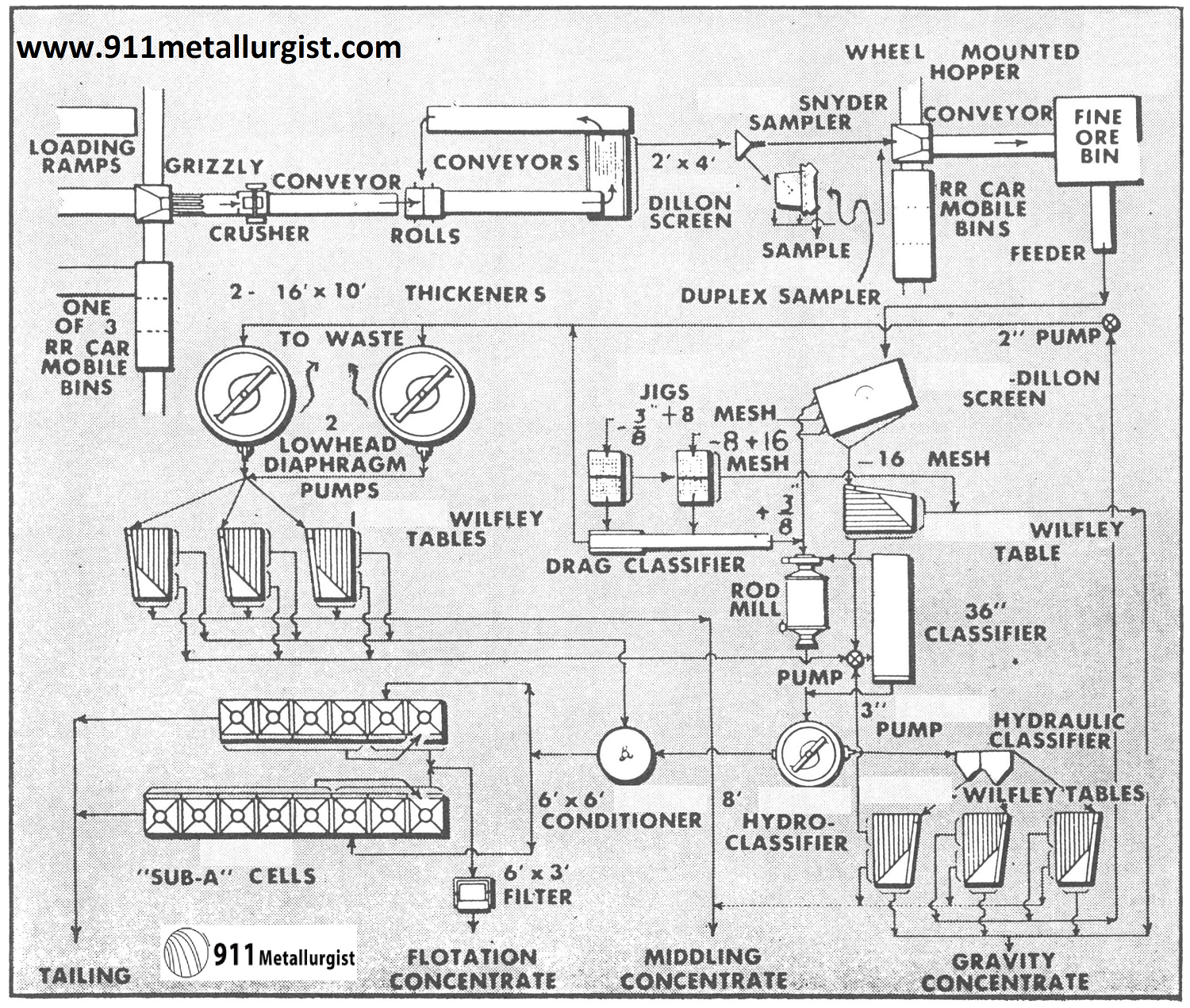
This flowsheet treats 100 tons of ferberite and scheelite tungsten ore per day. The sulfide minerals are pyrite, galena, sphalerite, chalcopyrite and molybdenum in minor amounts. Of the total tungsten recovered, 87% is obtained by the Mineral Jigs. Roasting and magnetic separation are employed to up-grade the final tungsten concentrates which assay 66 to 70% WO3. Normally a Mineral Jig is not recommended for handling a classified product as indicated in this flowsheet on the flotation tailing.
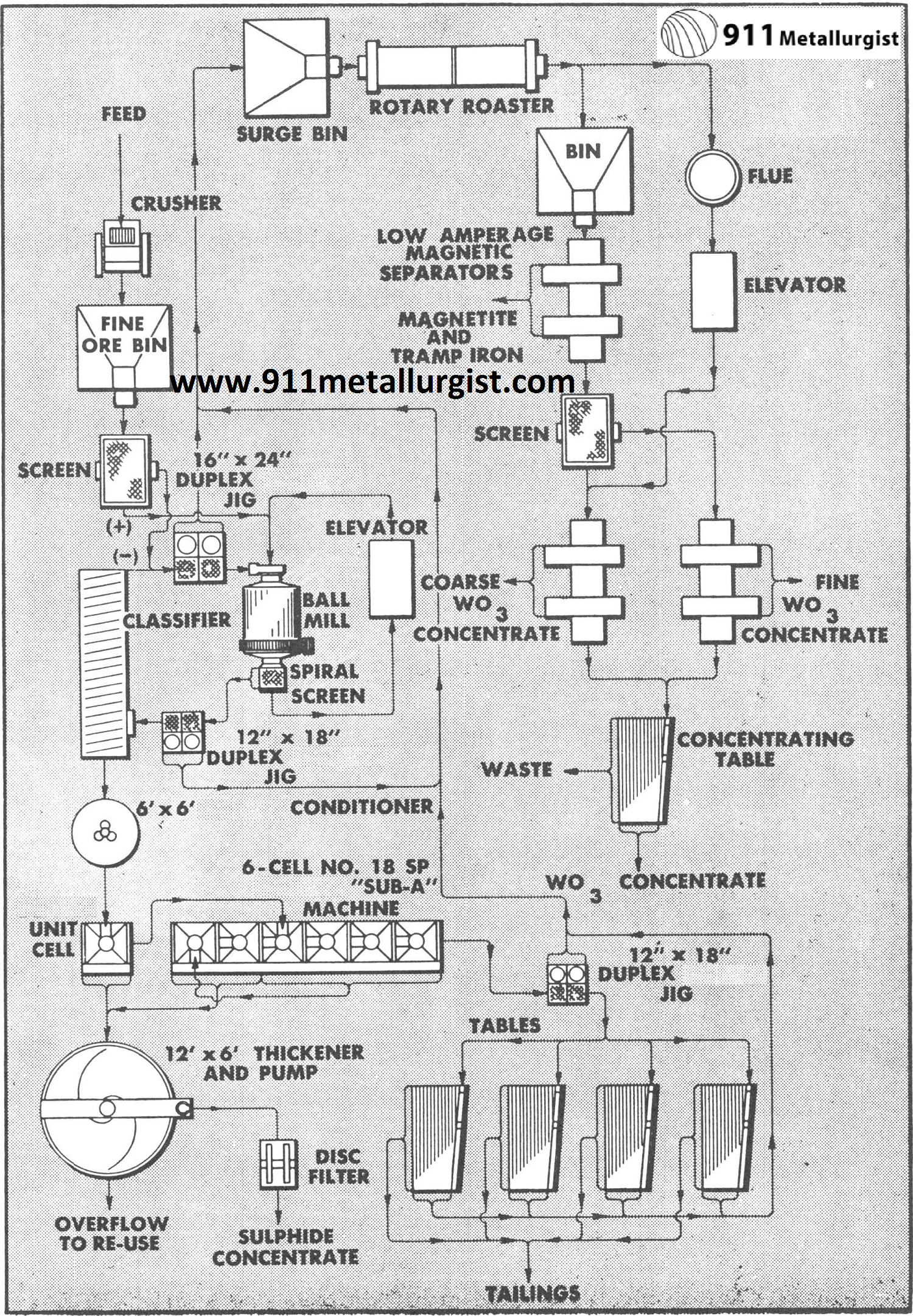
This flowsheet of a small Colorado operation recovers ferberite by an all gravity circuit making over 80% recovery. The Mineral Jig treats the minus sixteenth inch trommel screen undersize and is making a 60% WO3 concentrate. Gravity concentrates are heat treated and leached to remove phosphorus. When sulfides are present a Unit Cell is used on a batch basis to clean up the concentrate.
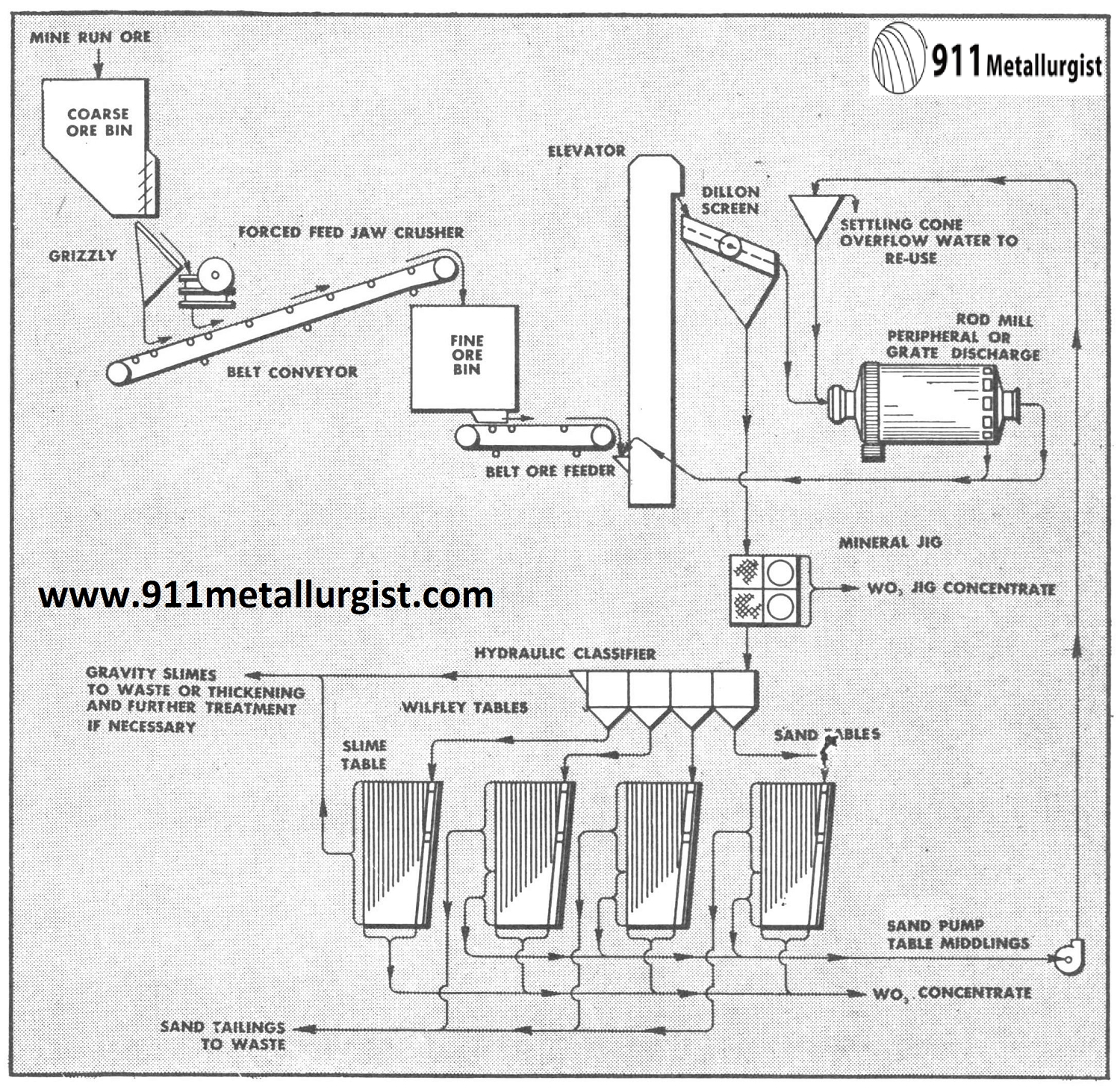
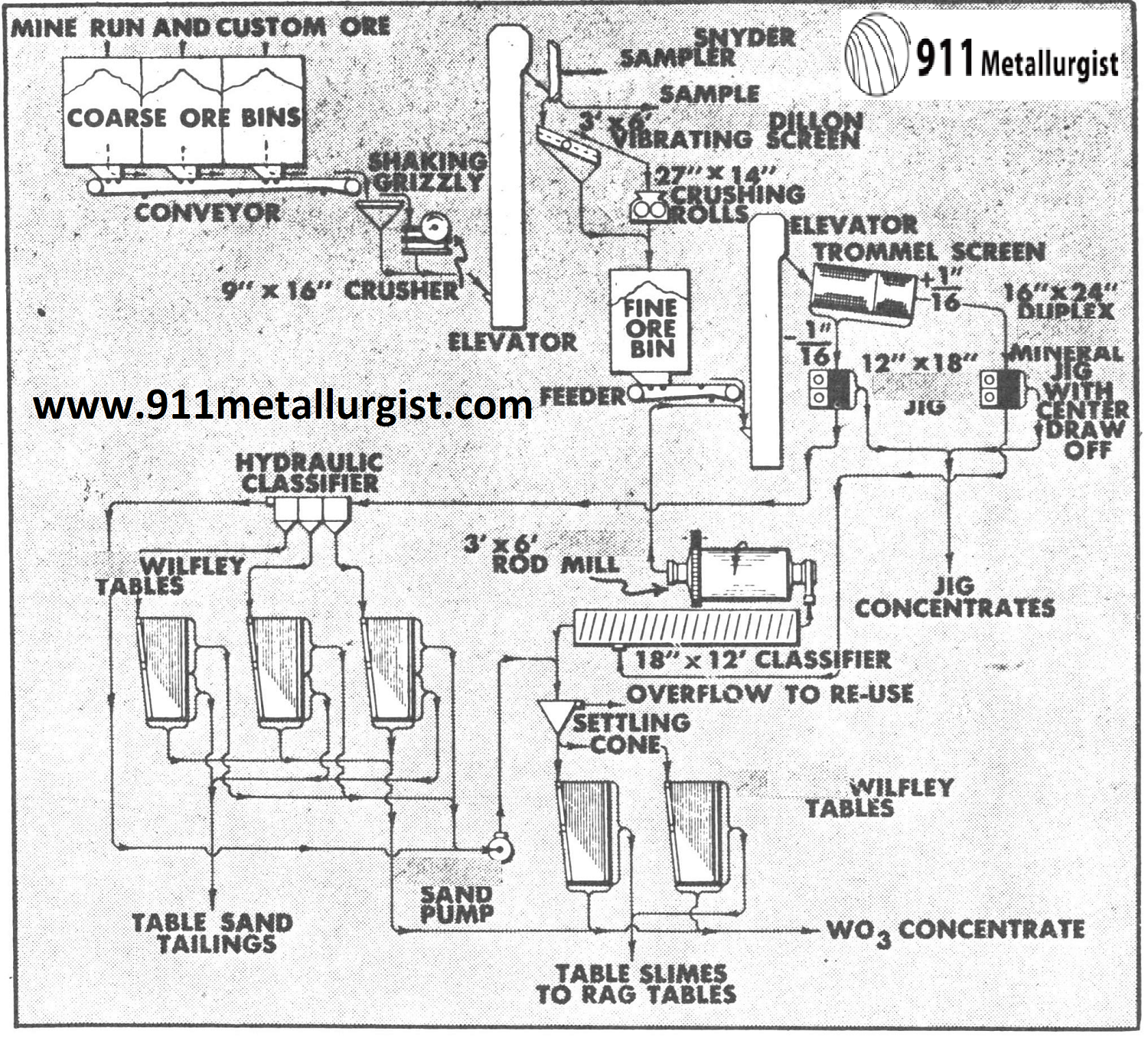
This all gravity flowsheet is applicable to many scheelite ores where crushing and grinding to at least 10 mesh is necessary to liberate the tungsten mineral. A 50 ton plant in Nevada recovered 94% of the tungsten when grinding to 22 mesh and producing a 60% WO3 concentrate from ore containing up to 2% WO3.
Recovery Tungsten by Gravity
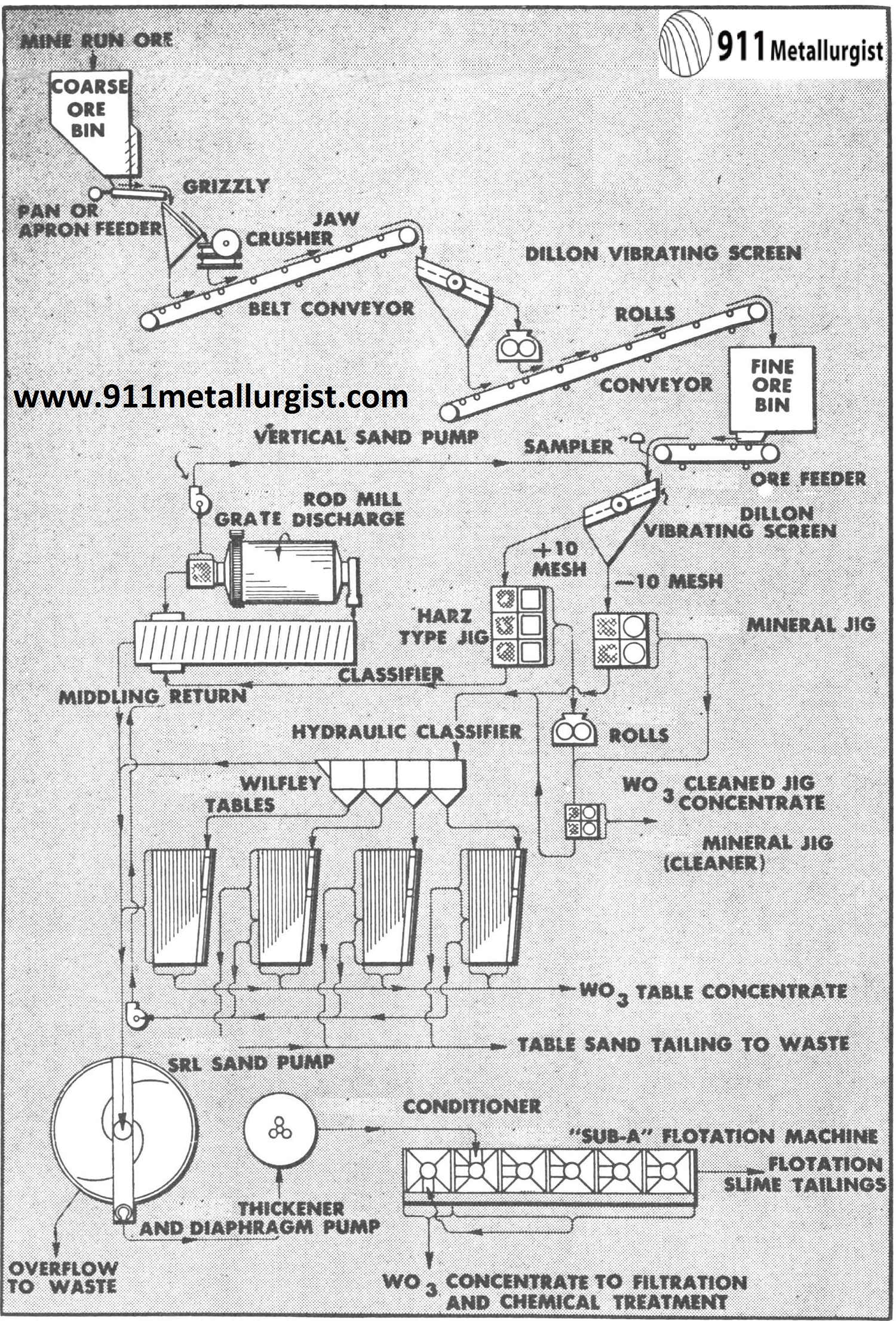
This is a typical flowsheet for tungsten ore containing little or no sulphide minerals. Both coarse and fine jigs are used because some of the scheelite is free and some is present as a middling in the coarser sizes. Roll crushing of the coarse jig concentrate and combining it with the Mineral Jig concentrate for cleaning is usually necessary. Tabling and flotation recovers a high percentage of the tungsten not saved by the jigs.
Recovery Tungsten by Gravity and Bismuth by Flotation
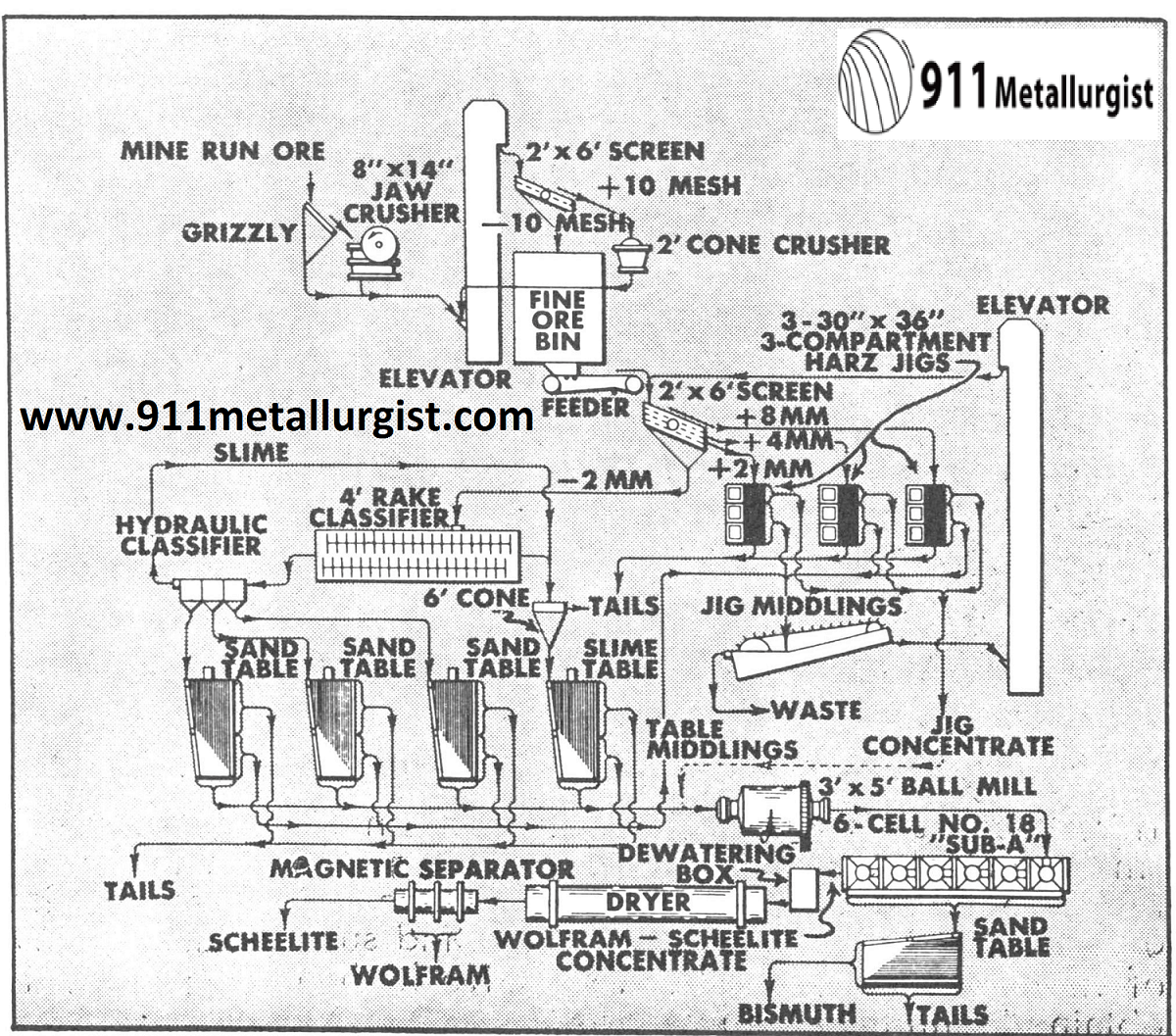
This 200 metric ton plant in Argentina treats a complex ore with about ½% WO3. This plant is being modernized and will include several Mineral Jigs plus flotation for bismuth recovery and sulfide removal.
Recover Fine Tungsten Slimes
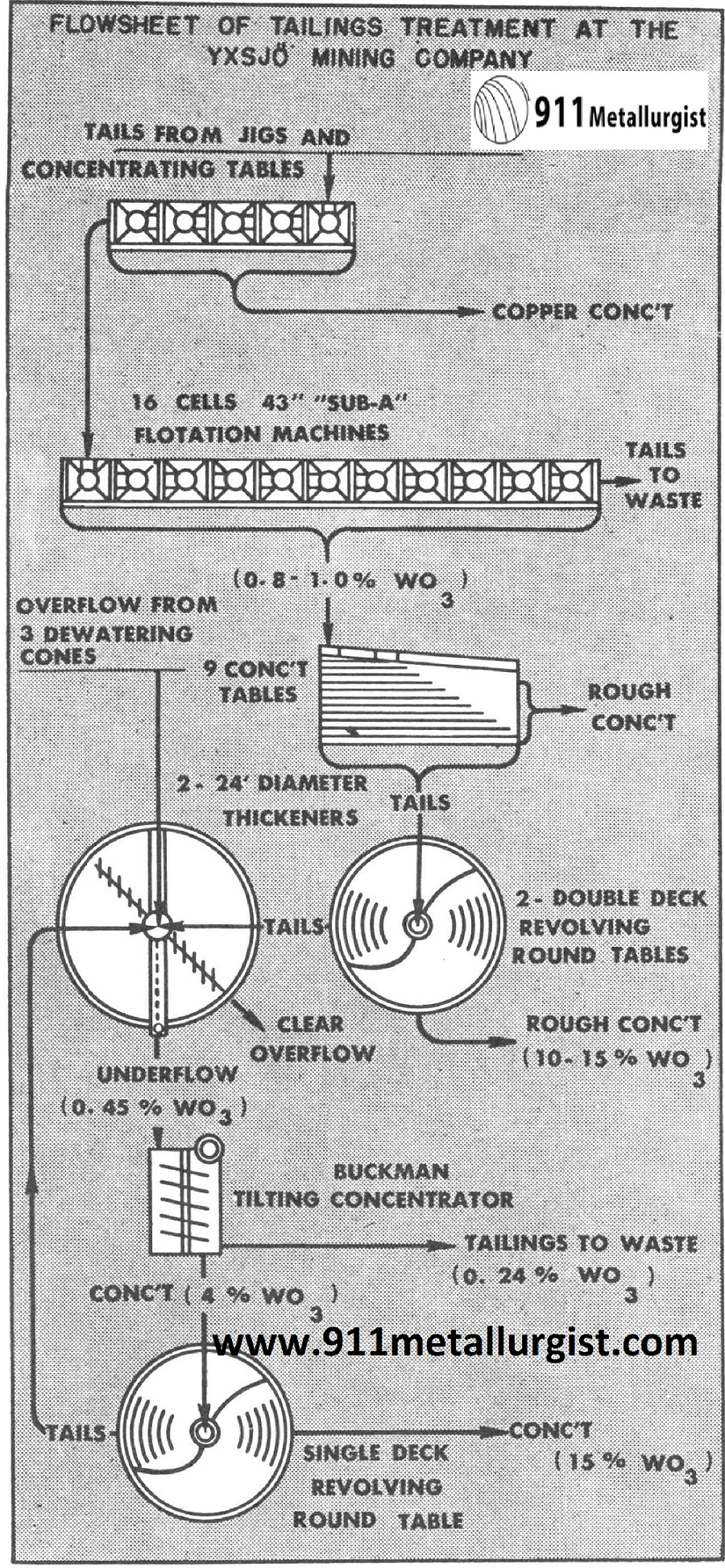
This plant in Sweden recovered 45% of the tungsten which was formerly lost. This was accomplished by the installation of the Buckman Tilting Concentrator for recovery of fine scheelite in the slimes.
Recovery Tungsten by Leaching and Flotation Method
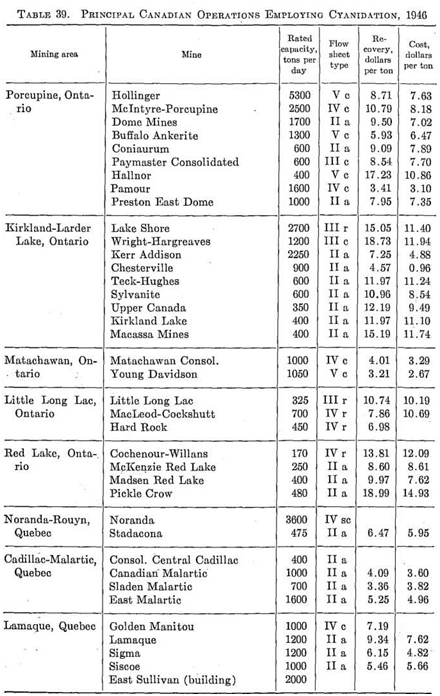
This Canadian operation removes gold and sulfides from gravity scheelite concentrate by leaching and flotation methods. Up to 65% of the tungsten recovered is obtained by the Mineral Jig—a product assaying 72-73% WO3.
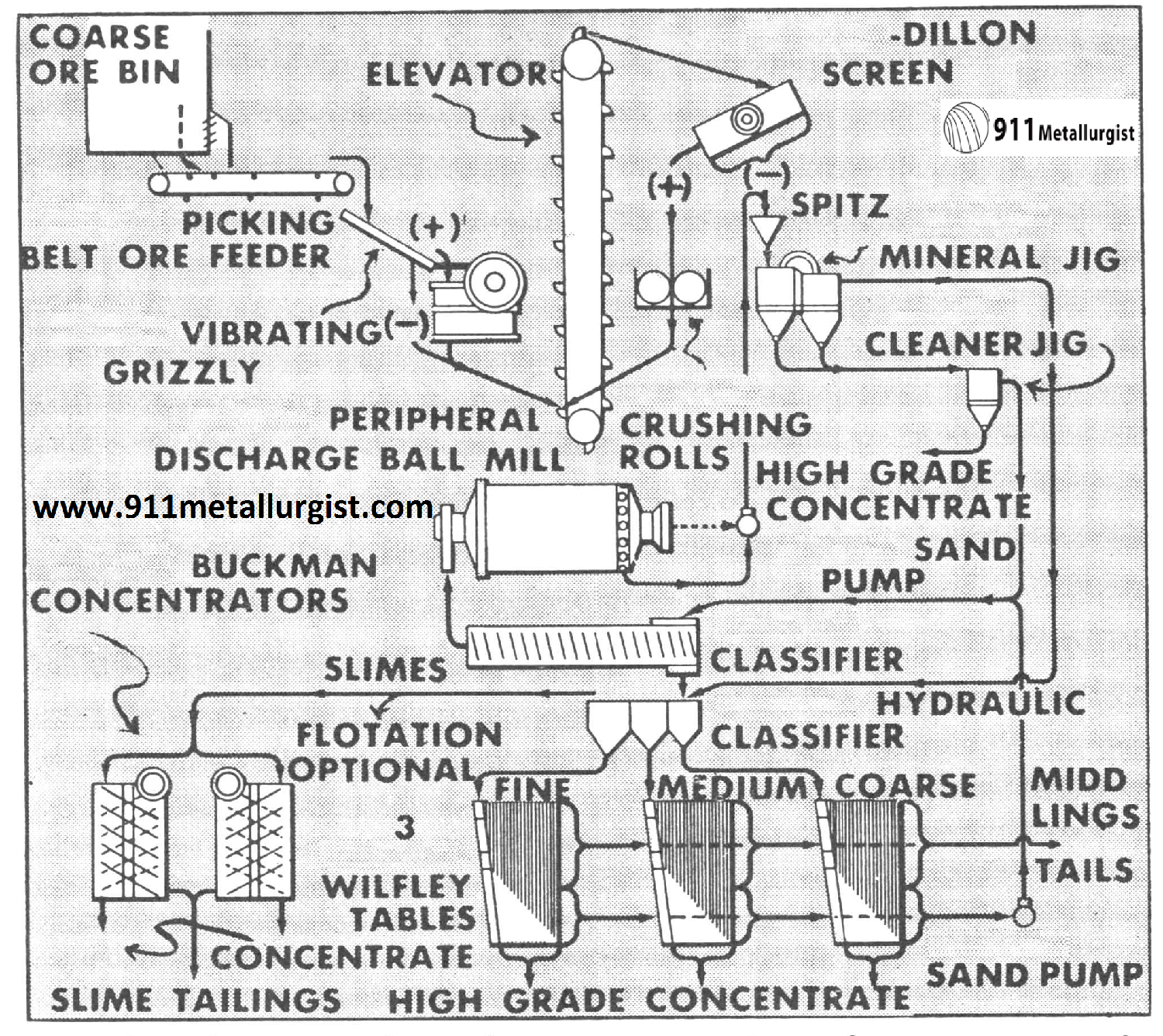
This flowsheet is adapted to concentration of tungsten ore in small tonnages. The Mineral Jig plays an important role due to its ability to handle an unclassified feed, with outstanding recovery in coarse sizes and substantial recovery of fine tungsten minerals. A selective high grade product is obtained with minimum water.
Tungsten Flotation
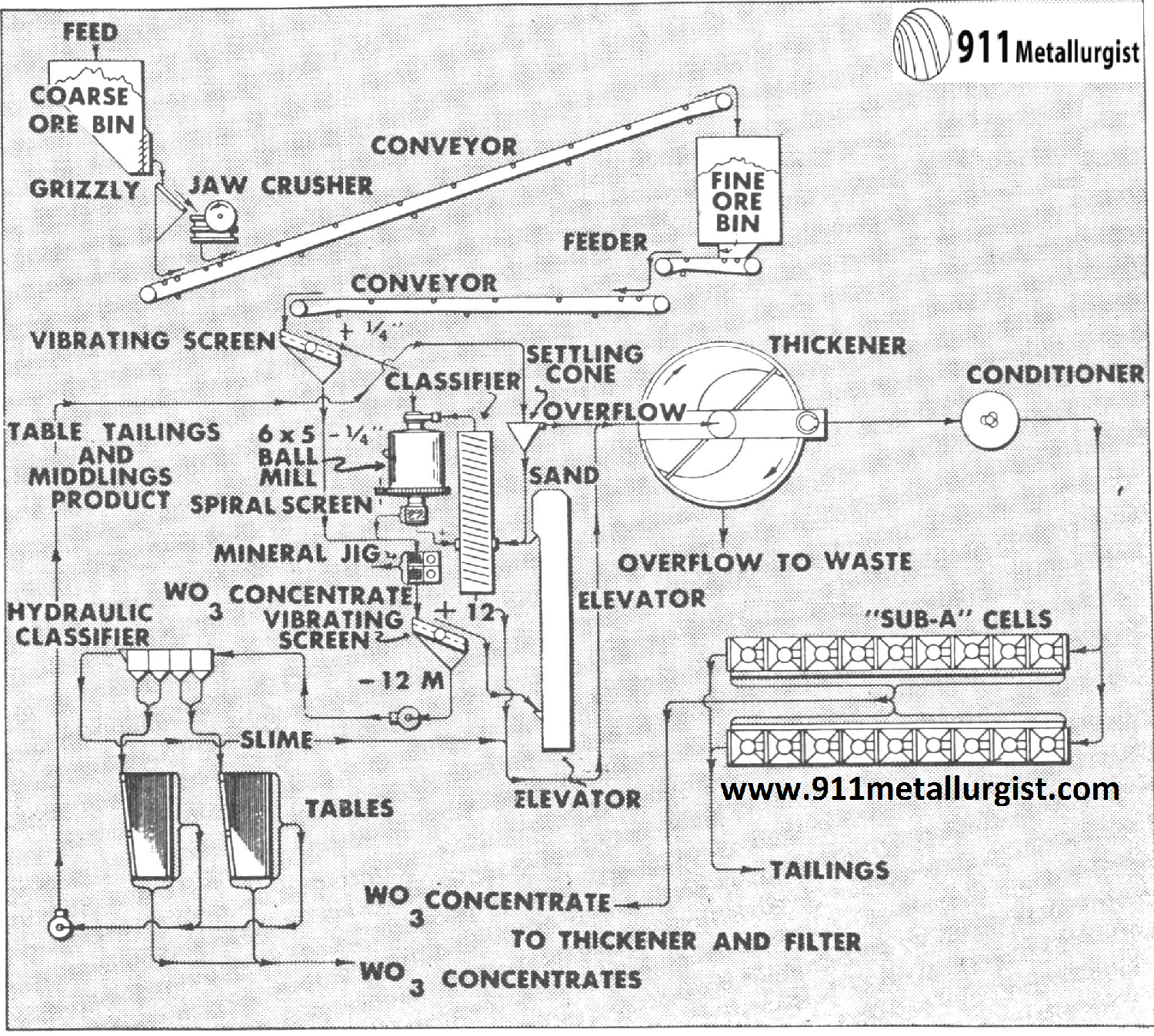
Flowsheet of a Nevada mill treating 150 tons per day scheelite ore. Flotation feed is all minus 65 mesh; reagents used for scheelite flotation are soda ash, sodium silicate, oleic acid, and reagent 708. Overall recovery of tungsten is plus 92%. Flotation concentrates are subjected to chemical treatment.
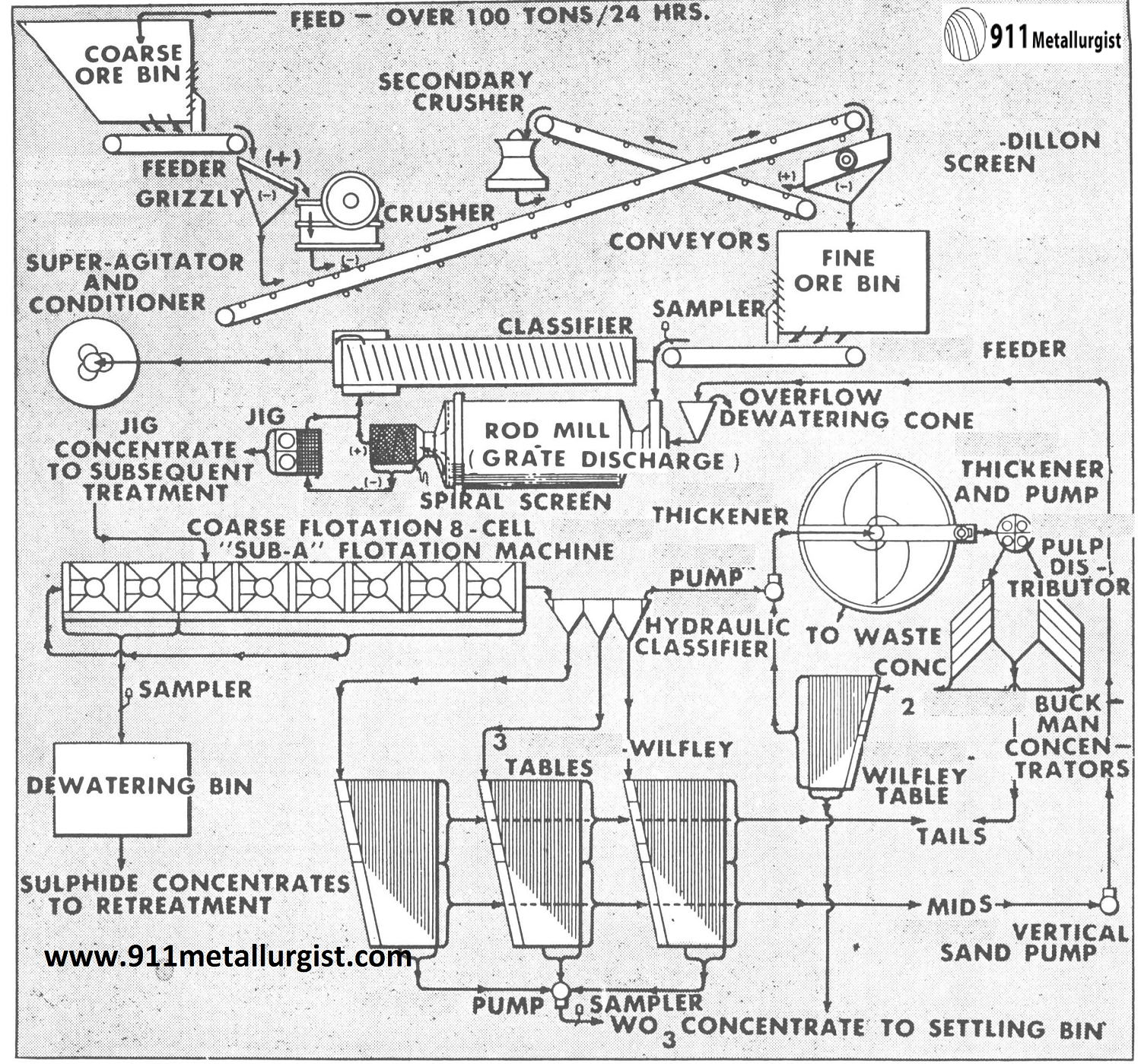
This is a typical flowsheet for a tungsten ore containing an important amount of gold associated with sulphide. The Mineral Jig recovers a high grade tungsten-gold product which is amalgamated and cleaned up in a batch through a Unit Cell for gold and sulfide removal. “Sub-A” flotation removes the balance of the gold bearing sulfides. Tables and the Buckman Tilting Concentrator recover the fine tungsten not saved in the jig.
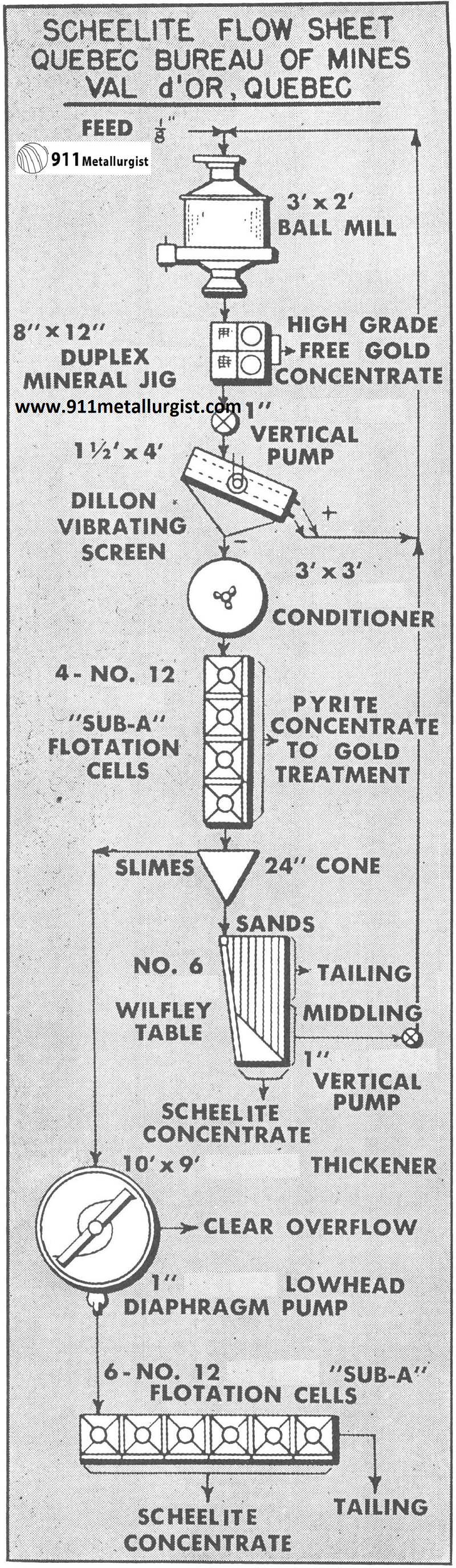
This flowsheet was used in the Quebec Bureau of Mines to test tungsten ores.
Ferberite Flotation
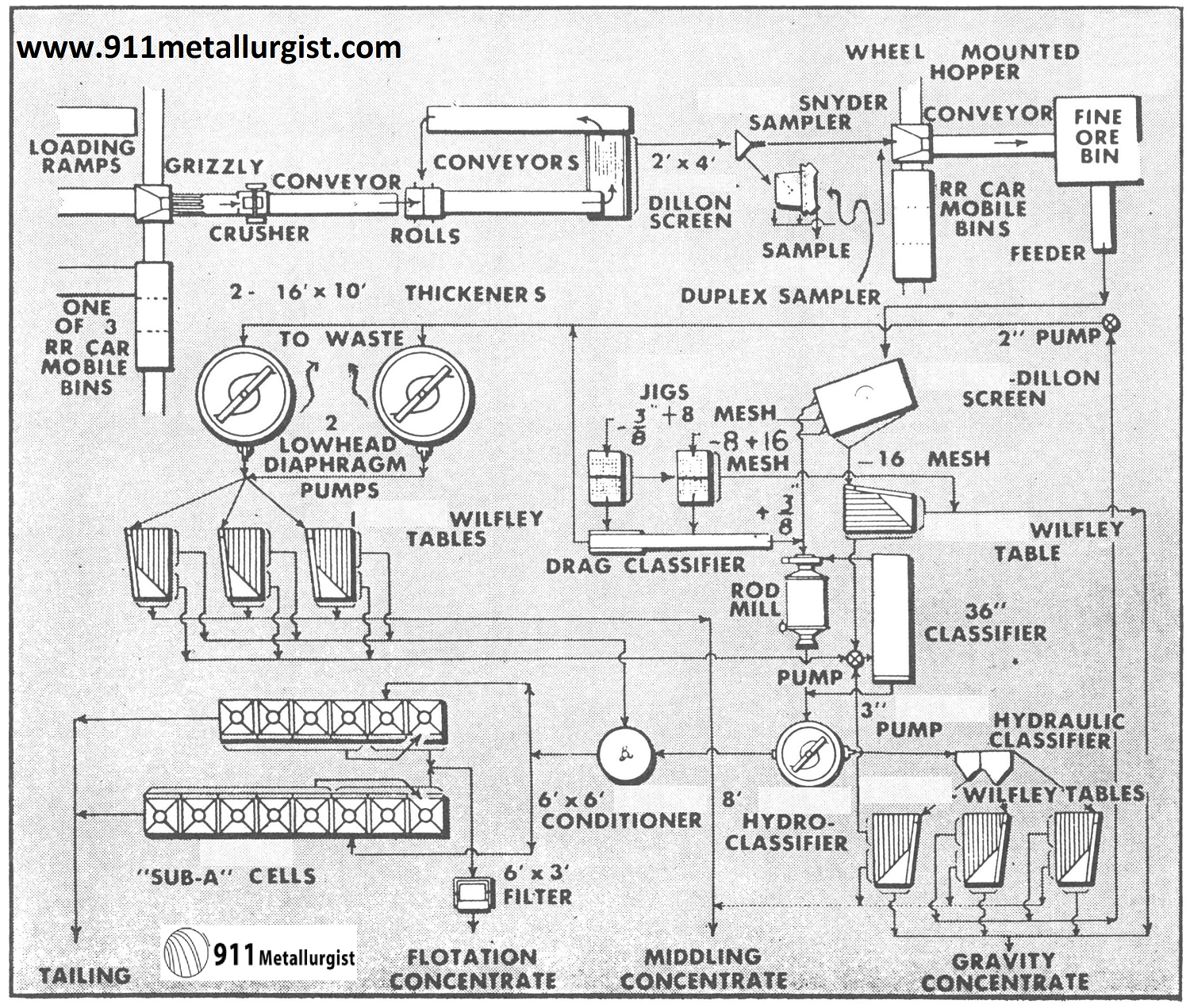
This Colorado Tungsten Mill handled ore, principally ferberite, on a custom basis during World War II. Stage crushing and gravity concentration were used. Concentrates assayed from 51 to 71% WO3. Low grade ferberite flotation concentrate was shipped to a chemical plant for extraction of tungsten.
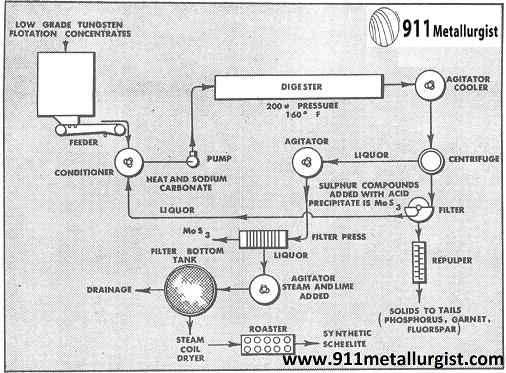
In one of the large California tungsten operations, scheelite is recovered by flotation. The resulting concentrate is deflocculated and tabled to recover the high grade cut. Table tailings containing slimed tungsten, phosphorus, fluorspar, garnet, molybdenum and tungsten is chemically treated to produce synthetic scheelite, CaWO4. In the flotation section the copper is floated as a sulphide product ahead of the scheelite flotation section after grinding the ore to all -65 mesh.
Jigging Tungsten
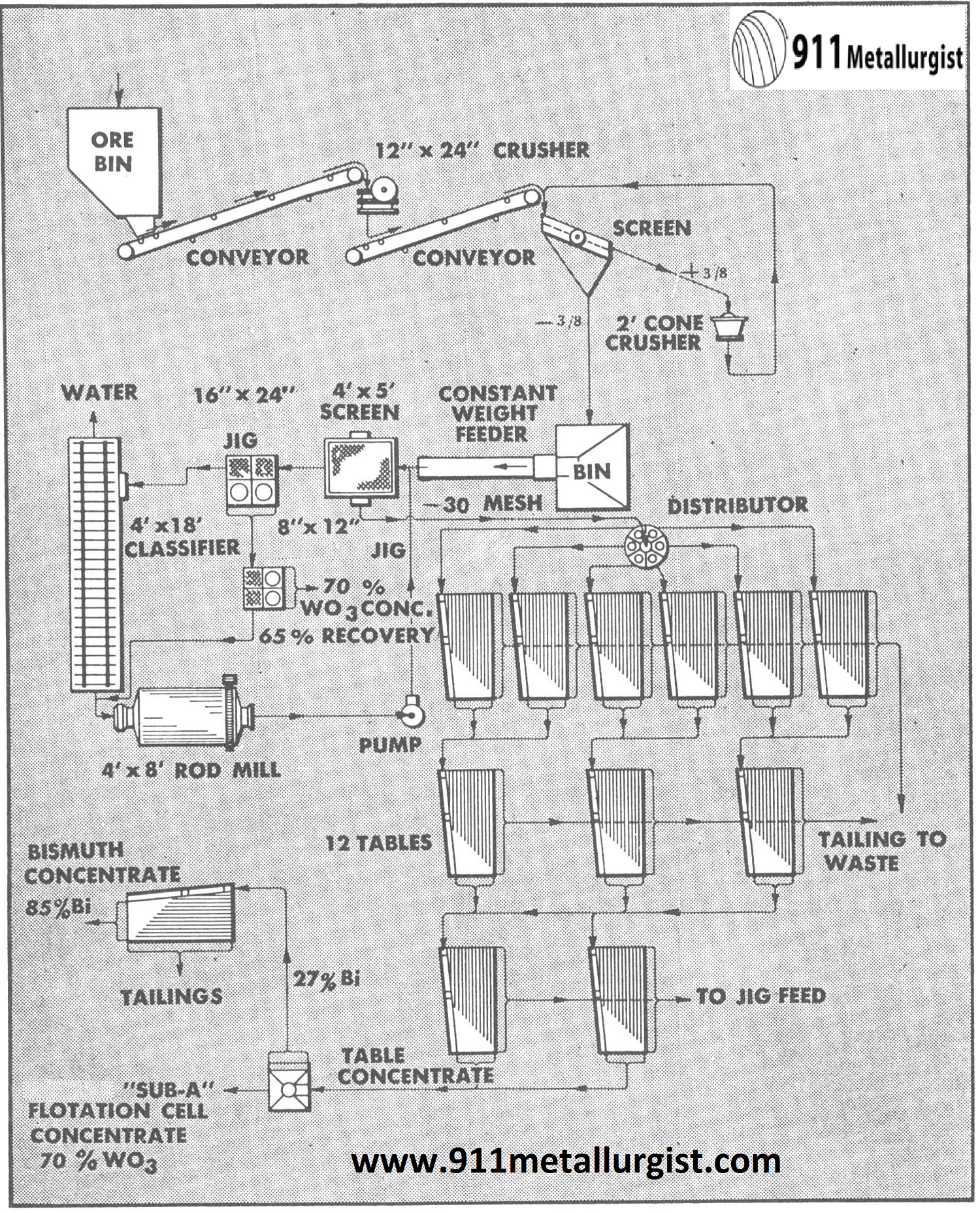
In this flowsheet of a South African tungsten plant the Mineral Jig recovers a substantial amount of high grade WO3 concentrate. This plant treats 110 tons of ore per day and total WO3 recovery is over 90%. Bismuth is removed by leaching the concentrate with nitric acid.
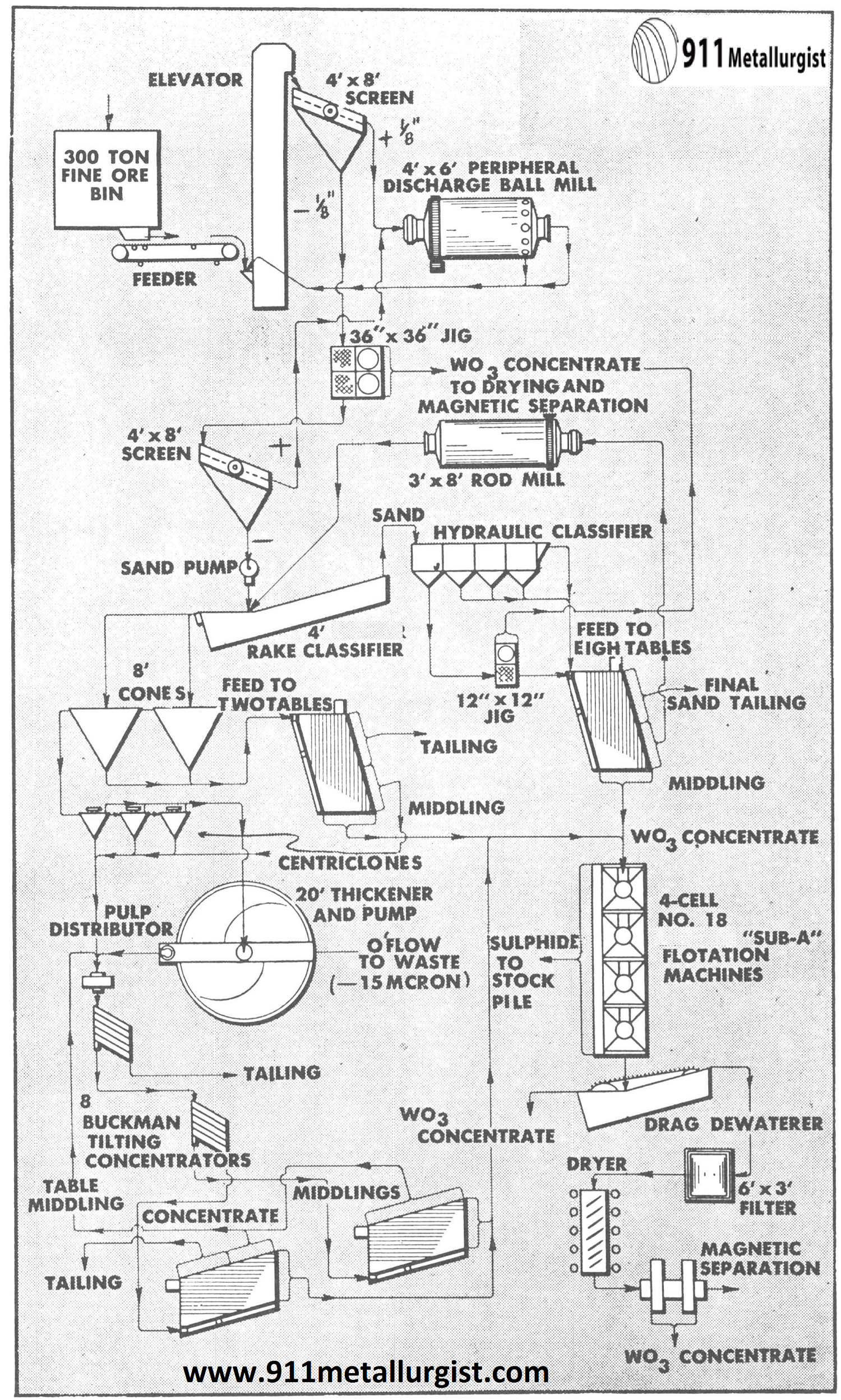
Flowsheet of one of the large U. S. tungsten producers treating a ferberite ore. The feed to the Buckman Tilting Concentrator is 205 tons per day and is -200 mesh + 15 microns. About 70-72% of the WO3 in this size range is recovered by the Buckmans.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
