 The most effective, and really only way for recovery of zinc sphalerite by froth flotation is by first activating the mineral with CuSO4 (Copper Sulphate) and allowing some conditioning time. How much time is optimum for effective zinc activation and good froth flotation?
The most effective, and really only way for recovery of zinc sphalerite by froth flotation is by first activating the mineral with CuSO4 (Copper Sulphate) and allowing some conditioning time. How much time is optimum for effective zinc activation and good froth flotation?
Obviously the answer to that is dependent on the test sample’s mineralogy, P80 grind size, pH and intensity of the conditioner/reactor used as well as maybe some other weirdo chemical interferences.
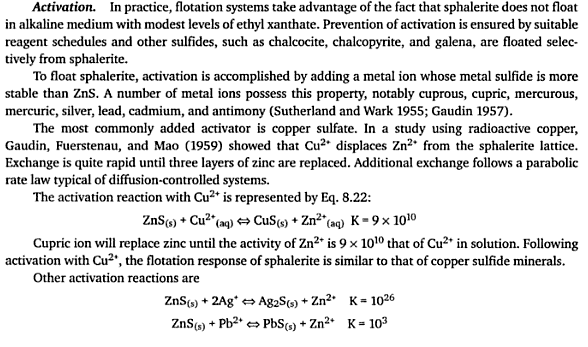
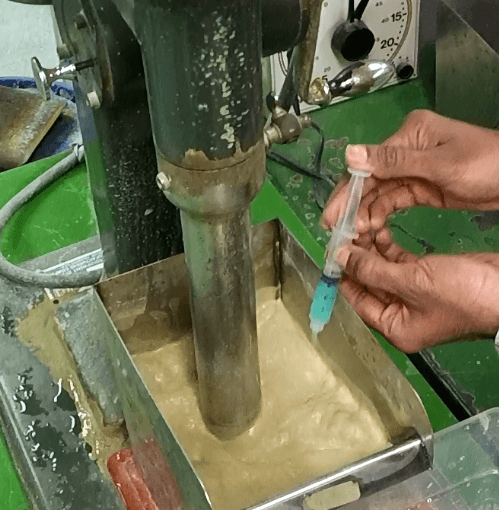
I ran a series of laboratory float tests at various conditioning time using a D-12 Flotation Cell as the reactor and running at 1500 RPM. The CuSO4 dosage is 3 cc (10%) for a 1 Kg charge on ore around 7% Zn and under 1% Fe for a dosage of some 300 gram per tonne (g/t).
The chemical reaction for sphalerite activation is explained below.
I conducted 4 tests using for conditioning time 5 minutes, 2 minutes, 1 minutes and 15 seconds. In all cases, after either of the minimum conditioning time, Zinc Sphalerite floats readily. All tests were performed in a 5 liter cell. These were rougher only from which 3 froth flotation concentrates were collected over a 10 minute time span.
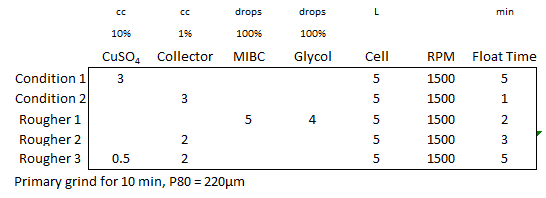
The laboratory results indicate a longer conditioning time accelerates zinc sphalerite flotation kinetics particularly in the first rougher concentrate. At the end, the final or terminal metal recovery appears unaffected by conditioning time.
What is clearly impacted, as theory predicts, is the lowered selectivity of the flotation system with less contitioning. The final recovery is the same, but the associated concentrate grade is lessen.
As conditioning time is reduced, the concentrate mass pull is increased in ordered to obtained the target recovery.
The rising Grade VS Recovery curves (green to black to red to blue) cleanly display the relationship CuSO4 Conditioning/Contact Time VS Zinc Sphalerite Recovery; the comparison of results is simple.
Flotation Activating Reagents
The outstanding example of an activating reagent is copper sulphate ; this is universally employed in the second stage of the separation of lead from zinc sulphides, in order to float the sphalerite after it has been depressed in the first or galena flotation stage. The reaction appears to be a chemical one, the zinc sulphide becoming filmed with copper sulphide ; its effect is to flocculate the mineral and make it floatable. Xanthate is almost always employed to intensify the flocculation.
The presence of lime or sodium carbonate, either of which will precipitate the copper as a hydroxide, has apparently no effect on the reaction ; it is common practice to put lime and copper sulphate into the circuit at the same point, the one to depress the pyrite and the other to activate the sphalerite. The addition of copper sulphate and xanthate together, however, is dangerous, presumably because of the formation of an insoluble xanthate of copper which has neither the activating nor the promoting properties of its constituents. The usual method is therefore to put the xanthate into the pulp at the latest moment.
Copper sulphate is often added to the circuit in the form of a solution, which is made up from the crystalline salt CuSO4.5H2O, and usually contains about 15% of CuSO4, the apparatus from which it is fed being constructed of some material not attacked by the solution. A better method, however, is to add it dry in the form of crushed crystals.
A less common activating reagent is sodium sulphide. This finds its principal use in the flotation of oxidized lead minerals, chiefly cerussite, which it coats with a film of lead sulphide, thus rendering them floatable. It is occasionally employed in a similar capacity to activate sulphide and native copper minerals that have become tarnished or oxidized. Its disadvantage is that any excess added over the amount needed for the chemical reaction has a depressing effect on all the minerals, making flotation difficult, if not impossible. Indeed, any soluble alkaline sulphide, or H2S, remaining in the pulp acts as a “poison” to flotation. The use of the polysulphide in place of the sulphide is rare. The consumption of sodium sulphide may be anything from 2 to 10 lb. per ton of ore.
Although the xanthates and aerofloat have been classed as promoters they may, at times, perform the functions of activators ; with some ores they are instrumental in rendering floatable minerals that would otherwise remain sunken.
https://scholar.lib.vt.edu/theses/ provides great papers on Zn Activation and Zn Conditioning by Copper Sulphate:
Studies of Activation of Sphalerite by Copper at Near Neutral pH