Table of Contents
Comparatively little trouble was experienced in gold milling with regard to the preparation of a pure bullion until the advent of the cyanide process. When ores were smelted the litharge method of elimination of baser metals and the subsequent parting had been worked out in fine detail. When alluvial gold was collected and smelted the product in gold and silver rarely contained five parts of base metal per 1000, yet some of this was introduced in the reduction of a little iron or other metal associated with the gold. The gold precipitated from chlorine solutions by means of charcoal gave fine gold running up to 998 fine, while that thrown down by sulphate of iron could be rendered equally pure by first precipitating the sulphate of lead, and allowing it to settle. Amalgam from batteries, if properly worked up, could be obtained containing only a few parts of base metal per thousand.
Precipitation on Zinc
On the introduction of the cyanide process and the zinc shaving method of precipitation, the purity of the resulting bullion was dependent on the metals and elements which passed into solution with the gold, and which were precipitated upon the zinc. It is undoubted that gold is one of the first metals to be thrown down from such a solution. If a fresh zinc box is taken, and gold-silver cyanide solutions allowed to flow through it, the gold is thrown down in the head box with a little silver, and the rest of the silver precipitated further on. Copper in the same way appears to be thrown out after the precipitation of the gold and silver has commenced;
the upper compartments at first contain but little, while the lower ones can be seen to be well coated. In course of time, owing to the mixing of all the material, this is lost sight of, but it would appear to be possible, were it worth while, to obtain precipitates of various grades by keeping the material from each compartment separate. Some such simple process will no doubt be discovered which will enable fine gold to be obtained as a separate precipitate from the silver.
The metals precipitated by zinc in alkaline cyanide solutions are gold, silver, copper, antimony, arsenic, mercury, lead, and occasionally nickel, cobalt, and cadmium. Selenium and tellurium are also dissolved in alkaline solution and thrown down. In addition to these elements, insoluble cyanides and ferrocyanides, sulphate of lime, carbonate of lime, silica, and organic compounds accumulate in the boxes. At first the precipitated metals adhere rather firmly to the zinc filaments, but in course of time the latter are eaten out, and the metals and admixed impurities become converted into a black mud; ferrocyanide of zinc, carbonate and other salts of lime tend to form a white crust, which on agitation makes a milky liquor.
The original method of cleaning up was to tease the zinc filaments out in a trough of clear water, passing only the fine muds through a screen. The coarser material was put back in the boxes, and the fine sediment settled in the trough. The precipitates were washed with water, dried and roasted in trays at a low red heat, the aim being to oxidise the zinc. The temperature should not rise above red heat, and the precipitates should be stirred to facilitate oxidation. Feldtman recommended the use of nitre in drying, so as to further oxidise metals present. Very large losses, however, have been caused by this, and this is no longer recommended. This method of open roasting with the volatilization of zinc oxide must have given rise to great losses in precious metals.
The roasted mixture was then smelted with borax, sand, bicarbonate of soda and fluorspar, in plumbago crucibles. After the charge first put in had melted, the slag was skimmed off, and a fresh charge placed on top of the bullion, and the operation repeated until the crucible is about half-full of bullion. The bullion was then poured. The fineness of bullion after this treatment varied from 600 to 900.
Samples of gold precipitates from the mine gave the following partial analyses. The letters A, B, C, D, E, F are compartments in one box:—
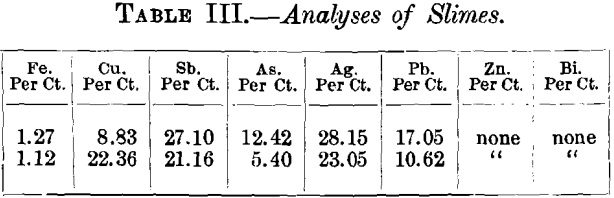
These slimes were obtained from each compartment as they fell from the zinc shavings in each compartment of the box. Since the box was not filled with fresh zinc before the samples were taken the analyses do not represent absolutely the rate of precipitation, but they indicate that the silver ratio increases towards the end of the box, also that the copper precipitation takes place near the end of the box. The large amount of siliceous material in compartment A was due to defective filtration. The slimes in the case are separated from the zinc. by being washed through fine gauze. They are dried by aspirating air through them, and when dry contain about 40 per cent, of bullion, the ratio of gold to silver being about 1 to 20. They are then roasted in trays and smelted in plumbago crucibles, with the addition of borax, soda, and fluorspar. The bullion runs up to 950 fine. In testing some of these slimes, after roasting, a considerable quantity of silver dissolved in dilute sulphuric acid, it is evident, therefore, that the high grade of this cyanide bullion is partly due to the oxidation of the products. It is worthy of note that while the gold and silver dissolved by the cyanide solutions from this mine is in the ratio of gold 50, silver 950, that derived from the amalgamated copper plates by amalgamation runs gold 500, silver 500.
Further reference will be made to the slimes from this particular mine in connection with other methods of treatment.
Usual Refining Method Now Adopted
At the Waihi mine a similar process for cleaning up was first adopted, but it has been found more economical to remove the zinc by dissolving it in dilute sulphuric acid and smelting the residual precipitate. The direct method of cleaning up has been abandoned almost everywhere, owing to losses through volatilization and dusting of the roasted precipitate, losses in smelting this powdery material, and losses in the slags, as well as the discomfort of having to smelt such a refractory material as zinc oxide.
In most places, not only are the slimes collected, but also the short zinc, and subjected to acid treatment. It is a great mistake to take this short zinc, since the cost of the zinc in the boxes is only increased by so doing. It is often stated that such short zinc is of no service in precipitating gold. This is not correct; if left until the next clean up the zinc present would be replaced by an equivalent amount of some metal or metals and slimes only would be left; as it is, such material is needlessly dissolved for the very small amount of gold still adhering to it. The boxes are cleaned up, starting from the top compartment, and the slimes and short zinc are sifted into a vat. The coarser particles of zinc, i.e., those not passing through a sieve of about 8 holes to the linear inch, are returned to the boxes.
The slimes are then allowed to settle, or at once passed through a small filter press. The solid material, or sludge, mixed with short zinc, is then transferred to a capacious vat, usually lead lined, and dilute sulphuric acid added in sufficient quantity to dissolve the zinc. The solution is kept so dilute that the sulphate of zinc will not crystallise out and prevent the action of the acid on the zinc. In Western Australia the consumption of acid is large, mainly owing to the amount of zinc unnecessarily removed from the box. The best plan is to allow the acid to be gradually added and to agitate with injection of steam. This will also serve to promote solution and to dissolve the zinc sulphate as fast as it is formed. During the period noxious gases are evolved, and should be carried away, either by means of a closed ventilating hood, which fits firmly on the edge of the vat, or else by a good draught in the open air. A certain amount of hydrocyanic acid, antimonetted hydrogen, arseniuretted hydrogen, and sometimes sulphuretted, or even hydrogen telluride and hydrogen selenide, is evolved by the action of the acid on decomposable compounds of these elements. The odors are distinct, and most disagreeable, with the exception of antimony hydride, which renders it all the more dangerous. It very often happens that the sulphuric acid used contains arsenic, and in this case arseniuretted hydrogen is sure to be evolved. The acid should be added until all the zinc has been dissolved. This can be readily tested by withdrawing some of the sludge, and after washing with a little water applying a dilute acid. If action starts then, more acid must be added. It is easy to adjust the supply so that there is very little acid in excess at the end of the operation.
The dilute sulphuric acid treatment only removes the zinc, since nearly all the metals precipitated in an alkaline solution
will remain in an acid one unaltered until the zinc has dissolved, with the exception of the small proportion of those converted into hydrides and so removed as a gas; cyanide of gold and silver are not decomposed, neither is the ferro cyanide of zinc. Calcium carbonate or calcium hydroxide is converted into sulphate.
The sludge now is filtered usually by passing it through a small filter press, preferably with gun metal frames. In addition to the ordinary filter cloth, filter paper and swansdown are used to retain the fine gold. Even when these precautions are taken some of this escapes into the filtrate. This is, or should be, sent to a sump, and allowed to settle for a long, time, or better agitated with a small quantity of lead acetate, and the precipitate of lead sulphate allowed to subside. It will carry down the finely divided gold. The precipitate is washed with water, and is then placed on shallow cast-iron trays, and introduced into an iron retort or muffle.
These simply consist of cylindrical pipes, open at one end, about six feet long, and 15 inches in diameter; a smaller pipe of four inches in diameter is tapped into the other end; this is led horizontally into the open air, and connected on to a vertical pipe, which above serves as a flue and condenser, and is prolonged below the horizontal pipe as a receptacle for mercury or other material which may condense and fall in the flue. The open end of the retort has lugs, and can be closed by means of a door, and bars resting on these. The precipitate is gently dried, and then heated to dull redness, and kept at that temperature for several hours. Most organic salts are destroyed, and the bullion usually assumes a light yellow colour. Very little stirring can be given to it for fear of dusting. This precipitate is then mixed with borax and sand, and smelted, usually about two parts anhydrous borax glass to one part of sand being added to 4 parts of precipitate.
The precipitate is usually smelted on large plants in Faber du Faur tilting furnaces. These are simply furnaces hung on trunnions, the crucible being placed inside, and in such a manner that it can be tilted with the furnace by means of a rack and pinion wheel, or simply by a hand lever.
Impurities
The bullion so produced varies in quality according to the nature of the ore from which it was produced. As a rule, at Kalgoorlie, it is more than 90 per cent, bullion, but in other places it is largely contaminated with copper, tellurium, selenium, antimony, lead, and other metals. If the precipitates could be smelted in fireclay crucibles the bullion would be several per cent. higher grade than when smelted in plumbago pots, but since the latter are, as a rule, much larger, and are much more reliable in the fire, the sacrifice of a few degrees of fineness must be made. The slags produced are clean
if they are siliceous and glassy, and fusible, but if stony and basic are often very rich, not only in prills of gold, but in gold either forming part of the slag or in such a fine state of division as not to be readily separated by crushing and panning or washing off the lighter material. The amount of slag varies with the composition of the precipitate. If through defective filtration siliceous slimes are allowed to enter the precipitating boxes, a larger amount of silica may have to be slagged off, and the weight of the slag may be several times the weight of the bullion recovered. In such cases losses through prills of gold remaining in the slags are heavy, and there is much trouble caused in smelting owing to the great bulk of material.
It may be safely said that unless the ore is high grade the weight of slag is greater than the weight of bullion, and the trouble of smelting is due to the melting and cleansing of this slag. The purity of the bullion is also largely affected by the admixtures present in the slag, some of which are reduced, and pass into the metal; others, such as calcium sulphate, are reduced to sulphides, giving a sulphide matte, which is often diffused through the slag, retaining a considerable quantity of gold. Whatever preliminary process is adopted for the elimination of such metals or compounds as tend to increase the amount of slag will not only give a purer bullion, but will lessen the cost of smelting. The ordinary mechanical impurities, such as finely divided ore or silica, should be scrupulously eliminated, and the solutions should be perfectly filtered or clarified before entering the zinc boxes. From the clear solutions silica, which has been dissolved in the alkaline solutions, will sometimes separate out on reaching the zinc boxes. In addition, calcium carbonate, calcium hydroxide, and, perhaps, some organic salts of calcium separate out as a white crust, also calcium sulphate from saturated solution; magnesium hydroxide also tends to separate out. Ferrocyanide of zinc also forms an incrustation on the zinc filament often in the form of small crystals. In addition to these earthy or siliceous compounds the heavy metals in the alkaline solution are thrown down. An impurity in the form of lead, in order to form a. couple with the zinc for the more effective precipitation of the gold, is now commonly introduced. Sometimes the zinc shavings are dipped in lead acetate, as suggested by J. S. Macarthur; more often the acetate is allowed to drip continuously into the head of the precipitating boxes, and sometimes the lead acetate is mixed with the roasted ore as a corrective for bad roasting; the alkaline sulphides still left in the ore thereby becoming converted into acetates, and the lead remaining as sulphide with the ore. In most cases, when sulphates are present in the neutral roasted ores, the excess of lead becomes fixed as a sulphate, but when slightly alkaline solutions are used it is likely that whatever fraction of the lead is not converted into carbonate or insoluble salt, passes on with the cyanide solutions into the zinc boxes. In some mines at Kalgoorlie, where the last method is practised, very little lead solution finds its way to the zinc boxes, since the gold precipitate is almost free from lead. In other cases, where lead solutions are actually brought into contact with the zinc it would appear to be a better plan to add these only in the rear end compartments of the precipitating boxes, or else in a small tail box at the end of the main ones. Owing to the precipitation of admixed metals in the head boxes, also the heavy precipitate of gold and silver the lead, which is rapidly thrown down, can not have more than a fractional value. Its full effect would become apparent if added so as to throw down the bullion, which would otherwise pass through the boxes in solution. By cleaning up this portion of the boxes occasionally, and keeping the bullion separate, lead would not become admixed with all the bullion to any marked extent. There is invariably some lead in the zinc shavings, and this becomes concentrated as the zinc dissolves, but the amount is small as compared with the gold and silver present. It is not possible in practice to prevent the precipitation of copper, antimony, arsenic, mercury, cobalt and nickel, tellurium and selenium from solutions containing them. It may be remarked, however, that very little tellurium is thrown down in the zinc boxes at Kalgoorlie, when the ores are roasted, and simple cyanide solutions are used; when, however, the bromo-cyanide solutions are used on raw ores a large quantity of tellurium is thrown down with the gold. At the Waihi mine (N.Z.) selenium is precipitated with the gold and silver on zinc from cyanide solutions used on raw ores.
Modification of Acid Treatment
It was found many years ago by the author that when calcium carbonate, or calcium hydroxide separated out on the zinc precipitate, when this was treated with sulphuric acid the calcium sulphate gave a lot of trouble in smelting. The removal of these compounds by means of dilute hydrochloric was successfully accomplished. The lime compound wholly dissolved before the zinc was attacked by the hydrochloric acid. It was further found that on teasing out the zinc shavings with the precipitate that the milky liquors remained after the bulk of the black precipitate had subsided, so it was only necessary to remove these to another vessel, and add dilute hydrochloric acid until the lime had dissolved, and filter this portion of the precipitate. After washing the precipitate remaining could be added to the bulky black precipitate, and the whole treated with the cheaper sulphuric acid.
This practice was also adopted by the Homestake Mining Co., U.S.A., the only difference being that the whole precipitate was treated first with hydrochloric, then washed before the sulphuric treatment was applied.
Use of Nitre Cake as a Substitute for Sulphuric Acid
An obvious modification of the sulphuric acid treatment for the solution of the zinc was the use of acid sodium sulphate. This material is largely produced in the manufacture of nitric acid, and the product known as nitre cake contains a large amount of it, and may be manufactured by having excess of sulphuric acid, to contain about 50 per cent, of sulphuric acid in a state which can be readily and safely transported, and which will, in solution, dissociate into sulphuric acid, and sulphate of sodium. The trouble with this material is its liability to crystallise when the free acid has been used up. Experiments by Messrs. Thomas and Williams have shown that the action of the acid sulphate containing the same percentage of sulphuric acid is slightly more efficient than the sulphuric acid itself. It was found that by diluting the solution until it contained 9 grms. of sulphuric acid per 100 cc of solution that the action on the zinc was vigorous, and that the acid was soon used up. After the action had ceased the spent liquor was run off, and more solution was used, until the zinc had dissolved. The result of bulk tests was so satisfactory that the method was adopted by the Simmer and Jack Company, S.A. There is little doubt but that this method of solution of the zinc, while it offers no chemical advantages over the use of sulphuric acid itself, yet may be of much advantage where the cost of sulphuric acid, owing to transport charges, is prohibitive. Only a dilute solution of sulphuric acid is needed, and this can be obtained from the salt as readily as from the acid itself. The obvious differences due to bulk of solution, crystallising of sodium sulphate, and longer time required owing to the solutions being necessarily more dilute than those required for sulphuric acid, do not affect the principle of the operations.
After treatment of slimes with dilute sulphuric acid, zinc will have become transformed to sulphate, but lead, copper, gold, silver, arsenic and antimony are still in the metallic state, any calcium present will have been converted into sulphate, and ferrocyanides of zinc will remain, and certain cyanides remain unaltered—silica also remains.
Experiments with Caustic Soda
Since zinc is soluble in caustic soda, according to the following equation:
Zn + 2Na OH = Na2 ZnO2 + H2
attempts were made to remove the zinc by this means. First strong solutions of caustic soda were used, and afterwards the product was heated to dryness strongly in iron pots. Not only was zinc removed, but silica and sulphides of antimony and arsenic are all rendered soluble. Lead is largely converted into peroxide; many organic salts were destroyed. In some instances it might be of advantage to use this method of cleansing the precipitate. If much silver is present it can be melted in a wrought-iron pot, and the slag, which is strongly alkaline, and easily soluble in water, used for neutralising solutions, instead of caustic soda. The zinc present is usually fixed by some element, such as a soluble sulphide; a small amount of plumbate of sodium forms, but, as before stated, most of the lead remains as a brown peroxide when the product is leached with water.
Distillation Process
The method of distilling out the zinc adopted in a few places has little to recommend it. The mistake is made of taking too much zinc from the boxes, and it is doubtful if the amount recovered pays for fuel and material. Further, unless the precipitates are free from admixed impurities, a dirty, honey-combed mass is left inside the retorts, all the metallic impurities are melted into the bullion, and there is considerable danger of loss through the zinc vapour carrying over gold. The latter danger is said to be overcome by the Sulman Picard process of mixing some coking material with the precipitate, which acts as a form of filter as soon as it has been coked for the zinc vapour.
