Compare NaCN Sodium Cyanide VS KCN Potassium Cyanide

There is probably no potassium cyanide now made for commercial purposes. With the development of the cyanide process the custom grew up, for economic reasons, of manufacturing what was called a “double salt” composed of potassium and sodium cyanides in varying proportions. This material was compounded so as to have a cyanogen content of 98% […]
List Sources of Cyanide Loss
Below is a list of things/factor which can cause cyanide loses in the leach process: The Precious Metals The amount of cyanide that combines with the gold in an average ore is negligible, but with silver it is different owing to the much larger quantity of metal that has to be dealt with to produce […]
Test for Traces of Cyanide
Here is a Qualitative Test Method to look for Cyanide Traces. To 500 to 1000 cc of the solution to be tested add 1 to 2 cc ammonium sulphide, (NH4)2S, and evaporate just to dryness. The final stages of evaporation should be done slowly. Cool, add 10 cc water, stir well, let settle, and filter. To the filtrate […]
Intensive Cyanidation

The upgrading of gravity concentrate for smelting purposes by use of intensive cyanidation is still in the experimental stage. It involves the leaching out of the gold into solution, using a strong cyanide mix, and then the electrowinning of that solution so as to recover the gold on the steel wool cathodes. This system is […]
Gold Leaching Plant for Sale

FLOWSHEET CY3, COUNTER-CURRENT DECANTATION CYANIDE PLANTThe following estimates are for counter-current decantation cyanide circuits and are based on average grade ore requiring average treatment time. These include precipitation and accessory equipment. The equipment required for a cyanide plant will vary considerably with the value of the ore, the settling rate, the chemical composition of the […]
Electrolytic Oxygen in Cyanide Solutions

There are two conditions generally prevailing upon the earth—those within atmospheric influence, tending towards oxidation, and those away from atmospheric influence, tending towards reduction. Practically all mineral substances from mines of any depth are in a reducing condition. Since the cyanide process, in order to dissolve silver or gold, requires that the prevailing conditions under […]
Gravity Recovery or Flotation ahead of Gold Cyanidation

Process Flowsheet NO. CY-3 is shown one of the outstanding improvements made in cyanidation whereby the coarse metallic minerals are removed from the grinding circuit by means of the Mineral Jig. The hutch product from this jig is amalgamated with a Amalgamator or Clean-Up Pan and thus the coarse mineral values, such as metallic gold, are […]
Counter Current Decantation Cyanide Gold Leaching

The Process Flowsheet CCD CY-1 shows (below) the continuous counter-current decantation system, in which all the ore is first reduced to a very fine state in the grinding mill-classifier circuit, in a cyanide solution. The slime overflow of the classifier, usually 70%—200 mesh, or finer, is sent to the first thickener, known as the primary thickener. […]
Gold Cyanidation Circuits

The principle of dissolving gold and silver values in solutions of potassium or sodium cyanide is old and has been thoroughly carried out in practice for many years. There have been only a few changes in the chemical procedure and also few changes in the type of equipment used. The gold and silver are dissolved […]
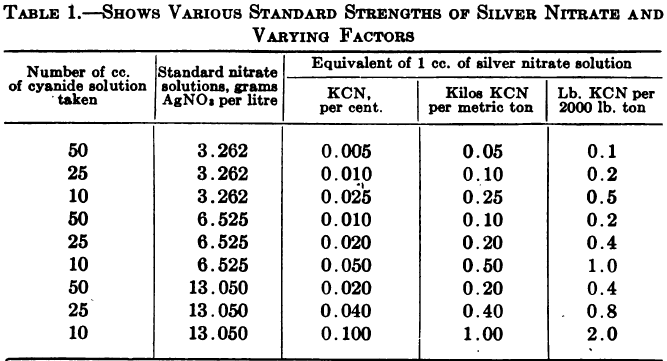
Estimation of Free Cyanide using Iodine and Potassium

The process of estimating free cyanide depends upon the fact that when a solution of iodine in potassium iodide is added to a solution of a simple cyanide, the reddish-brown color of the iodine solution disappears so long as the cyanide is in excess, since the reaction results in the formation of an iodide of […]
