Thiocyanide Thiocyanate Sulphocyanate Assay Determination
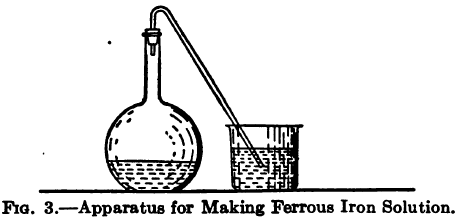
The method usually given for detection of Sulphocyanate (Thiocyanide, Thiocyanate) is to acidify and add a ferric salt, a blood-red color indicating the presence of KCNS. Silver and copper, however, interfere, and if present in sufficient amount may entirely prevent the formation of the characteristic color, so they must be removed before any determination can […]
Cyanate Determination Method
Clennell gives among other cyanate assay methods the following based on the fact that when KCNO is treated with an acid it does not decompose into HCN but reacts in the sense of the equation, KCNO + 2HCl + H2O = KCl + NH4Cl + CO2 By determining the amount of ammonium compound formed a measure […]
Determination of Ammonia and Ammonium Salts
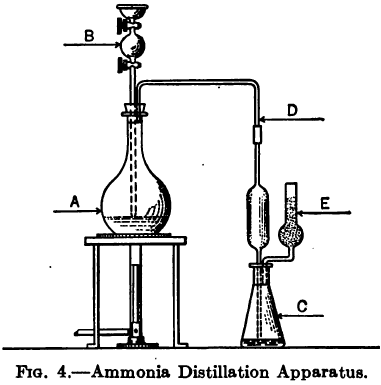
The following method is given by Clennell to determine the Ammonia and Ammonium Salts content by assaying cyanide leach solutions. “Add excess of AgNO3, i.e., sufficient to precipitate all cyanogen compounds, then a little NaCl to remove excess of AgNO3, filter, wash, evaporate filtrate on a water bath to a moderate bulk, distil with caustic soda or sodium […]
Volhard’s Silver Determination & Argentometry
Volhard’s method is a type of Argentometric titration of silver content determination. The Volhard Method requires: a decinormal ammonium or potassium sulphocyanate solution, a decinormal silver nitrate solution, ferric indicator. A decinormal silver nitrate solution contains 16.988 grams of AgNO3 per litre, or 10.788 grams of Ag. It may be made up by direct weighing of the pure […]
Determination of Acetates
Julian and Smart state that cyanide solutions undergoing decomposition form not only formates but acetates. Clennell states that he has never found acetates present in a working cyanide solution unless lead acetate was being used, but on the assumption that acetates may perhaps be formed in certain circumstances he suggests the following equation. 4KCN + 5H2O […]
Method to Assay for Soluble Sulphides in Leach Solution
This is seldom called for, but it happens occasionally that an appreciable amount of sulphide is developed in the cyanidation of concentrate, and some sulphide is sometimes apparent in the press effluent from aluminium precipitation which it may be desirable to estimate. For such small amounts Clennell recommends the colorimetric method. For this purpose a […]
Determine Oxygen Content in Leach Solution

Here is a way how to determine the oxygen content during gold leaching and Estimation of available Oxygen Content in Cyanide Solutions. Until recently no simple and reliable method has been known for the estimation of free oxygen in working cyanide solutions so that the one here described supplies something that has long been needed. The matter […]
Assay Method of Gold or Silver in Leach Solution
Gold & Silver Assay Method No. 1 Evaporate a known quantity in a square boat made of lead foil, scorify, and cupel. This is not reliable for low grade solutions because the amount that can be taken for assay is too small. Gold & Silver Assay Method No. 2 Evaporate a known quantity in a large porcelain dish whose […]
Principles of Gold Cyanidation Explained
What is commonly known as the Cyanide Process has for its object the commercially profitable recovery of gold and silver from their ores, and this is accomplished by the solvent action of an alkaline cyanide solution on the precious metals. The first step in the Cyanidation process is to mill or grind the ore to […]
Chemistry of Cyanidation of Gold & Silver

The usual reaction given for the dissolution of gold and metallic silver in cyanide solution is known as Elsner’s equation. 2Au + 4KCN + O + H20 = 2KAu(CN)2 +2KOH Silver sulphide, the form in which silver most commonly occurs in its ores, involves a different set of reactions, which are usually expressed thus: First […]
