Cyanide Poisoning Antidote

In 1910, the Committee of the Chemical, Metallurgical and Mining Society of South Africa, appointed to investigate cyanide poisoning, recommend as an antidote to Cyanide Poisoning the following: Thirty cc of a 23 per cent, solution of ferrous sulphate. Thirty cc of a 5 per cent, solution of caustic potash. Two grams of powdered oxide of […]
Agitated Cyanide Gold Leaching Test
In the old days laboratory tests were usually made by mechanically shaking up in a bottle for a given time a charge of ore and cyanide solution. The most generally convenient device for this purpose is a wheel, to which are attached boxes, each capable of containing a standard acid bottle, and with means for […]
Determine the Proper Grind Size for Gold Ore

Without mineralogy, estimating the optimum Grind Size for Gold Ore Sample is most conveniently made by Laboratory Testing and the agitation leaching method, and it will be necessary to make up 3 or 4 bottle charges in order to have enough ore* for the subsequent screen analysis. Take an average sample of the ore and grind […]
Calculating Cyanide Consumption
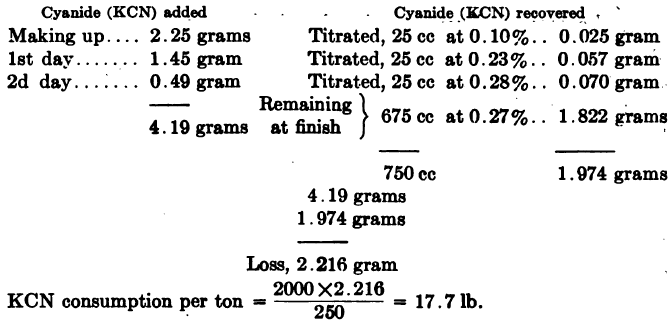
Computing Cyanide Consumption of a laboratory leach test may be done as in the following example: Ore taken, 250 grams. Ratio of solution to ore, 3:1 = 750 cc: 250 grams. Cyanide strength, 0.3% KCN. Or when working on the metric system, Ore taken 200 grams, ratio of solution to ore 3:1 = 600 cc: 200 grams, […]
Cleaning & Melting Gold Precipitate
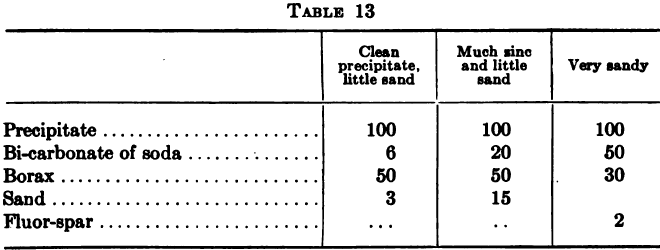
With zinc shaving precipitation the usual method of cleanup is to shut off the flow of solution and starting at the head compartment of a box to wash the shavings gently in the solution avoiding any action that would tend to break up the attenuated threads into short pieces. The zinc is then removed and […]
How Arsenic, Antimony Silver Compounds Affects Leaching
Ores containing silver in Arsenic and Antimony combinations offer considerable difficulties in cyanidation and it has usually been considered that the removal of these compounds by concentration was the only way to deal with them. G. H. Clevenger, however, when working on the ores of the Nipissing Mining Company of Ontario, Canada, found that by […]
Sulphide concentrate leaching

The question as to whether concentration shall be included in the treatment of a given ore will often depend on the possibility or otherwise of recovering the precious metals from the concentrate at the mine. It may be suggested that if the concentrate can be cyanided after being separated from the gangue, why can it not […]
Effect of Manganese on Silver Leaching

In Mexico and elsewhere there are large bodies of valuable silver ore containing manganese which present great, and hitherto insuperable, obstacles to a satisfactory extraction by cyanide. The proportion of the silver that is soluble in cyanide varies greatly and may be as low as 5% only. The mere presence of manganese in the ore […]
How Arsenic Affects Cyanidation Leaching of Gold
The Influence of Arsenic in the Cyanidation of Gold Ores is explained by W. B. Blyth has he is of the opinion that the presence of arsenic in an ore does not necessarily cause that ore to be refractory to cyanide treatment. He thinks the idea arose from the fact that arsenic is so often accompanied by […]
Metallurgy for Recovering Gold from Telluride Ore

Gold ore associated with Telluride is hardly soluble in ordinary cyanide solutions and special treatment is necessary for its extraction. There are two methods in use in such cases. Roasting The ore is ground dry to about 30 mesh and roasted; it is then ground to a slime in water or cyanide solution, usually in grinding […]
