Assaying for Cyanide
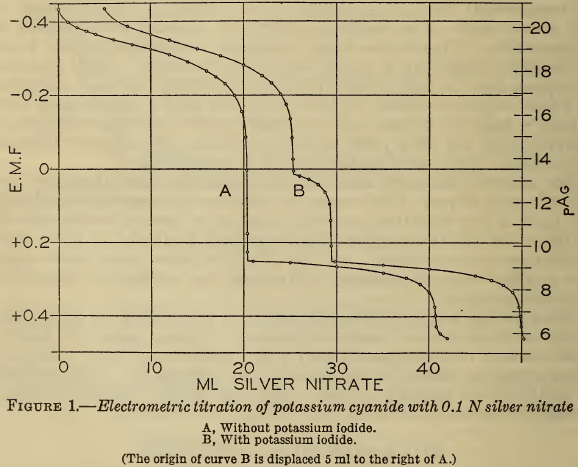
Methods for the analysis of cyanide silver-plating solutions were studied, including the determination of free cyanide, total cyanide, carbonate, chloride, ammonia, silver, iron, copper, and mercury. Electrometric titrations showed that the Liebig method for alkali cyanide is correct to better than 0.2 per cent. Addition of iodide makes the method still more accurate and overcomes […]
FREE Cyanide Assay Method
A simple example of the application of a complexation reaction to a titration procedure is the titration of cyanides with silver nitrate solution. When a solution of silver nitrate is added to a solution containing cyanide ions (e.g. an alkali cyanide) a white precipitate is formed when the two liquids first come into contact with […]
How to Collect a Sample for Cyanide Analysis
It is important to make use of composite sampling and to take at least five samples. Grab samples may be representative of flow during a short period and any other sample shown to be representative of waters being sampled may be applicable. The collection of samples for cyanide determination will require treatment to preserve the […]
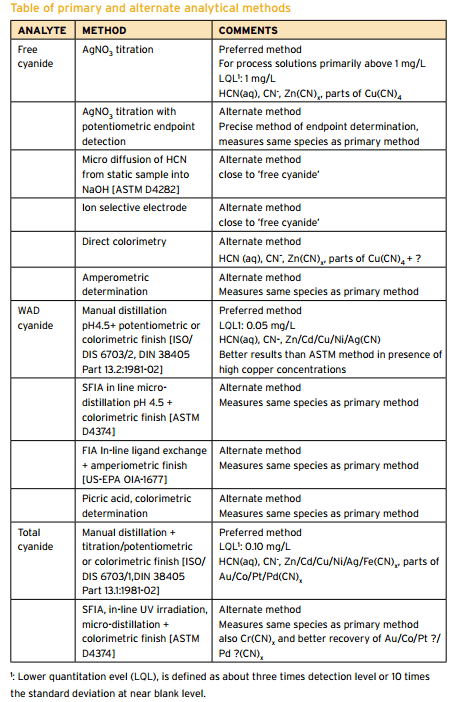
Cyanide Assay Methods
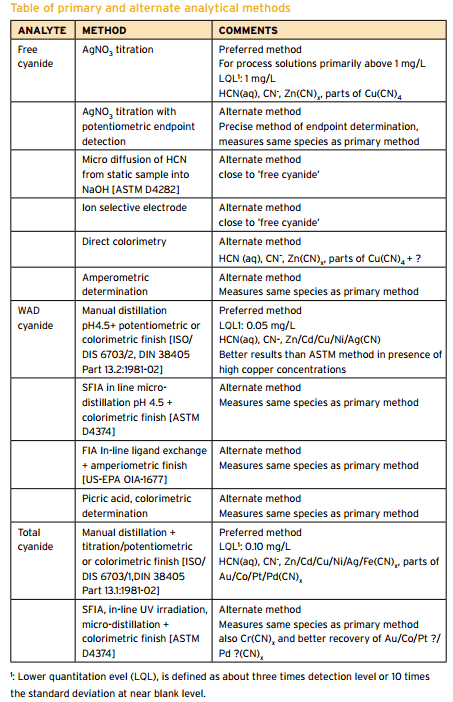
Summary of CN Analysis Methods Description Name Method Description Potential Interferences Measurement Free Cyanide ASTM D 4282 Passive Diffusion at pH 6 and room temperature • Storage • Measurement • Manual chlorination of cyanide with chloramine-T and subsequent reaction with pyridine-barbituric acid. • Maximum absorbance is determined by manual colorimetry. ASTM D 7237 Flow Injection […]
Cyanide in Nature
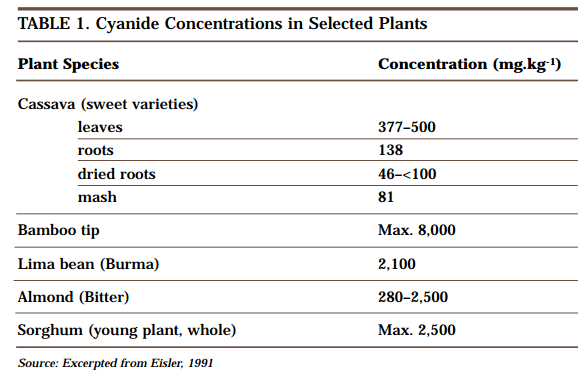
Carbon and nitrogen, the two elements that make up cyanide, are present all around us. Together they make up almost 80% of the air we breathe, and both are present in the organic molecules that are the basis of all life forms. Hydrogen cyanide was formed in the earliest stages of the development of our planet as a […]
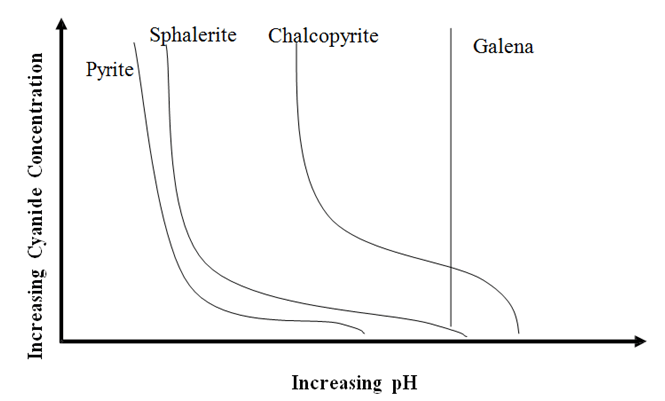
Effect of Cyanide on Depression of Sulphide Minerals
At the dosage of collector (not shown but relatively low), it is indicating galena will not float above pH 10.4, and it is not affected by NaCN. What you have to be careful about is that these lines all move if you increase or decrease the collector dosage. For example, you can get pyrite to […]
Estimation of Free Cyanide using Iodine and Potassium

The process of estimating free cyanide depends upon the fact that when a solution of iodine in potassium iodide is added to a solution of a simple cyanide, the reddish-brown color of the iodine solution disappears so long as the cyanide is in excess, since the reaction results in the formation of an iodide of […]
Why Oxygen is Important in Cyanidation
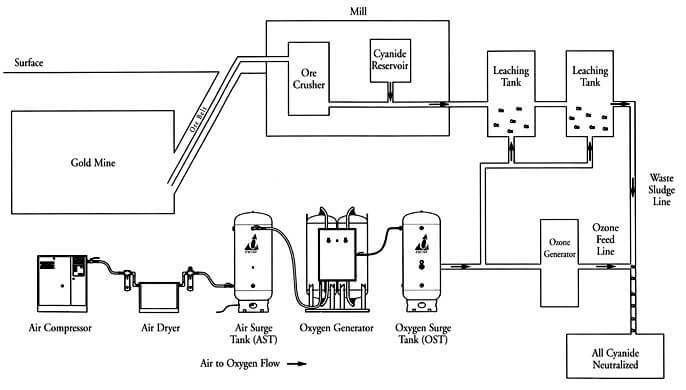
The Rule and Function of Oxygen in Cyanidation.—Oxygen appears to be an indispensable factor, either directly or indirectly, in the dissolution of gold and silver by cyanide solutions, except in the case of the haloid compounds of silver. Whether the action of oxygen in the dissolution of gold be a direct one, as illustrated by the […]
Copper Lead Concentrate Separation
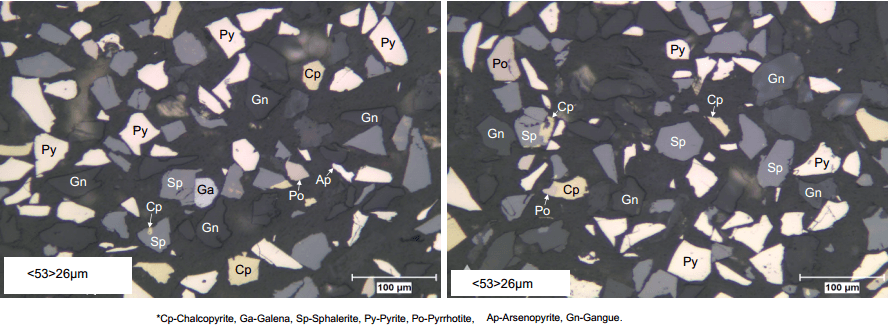
The ways to obtain successful Separation of Copper and Lead into individual Concentrates, several process approaches can be examined; bulk copper-lead flotation with a reverse flotation stage depressing copper, the same bulk flotation with a reverse flotation to depress lead and a sequential copper-lead-zinc flotation circuit as well as a simple cleaning of the bulk Cu/Pb concentrate by Pb […]
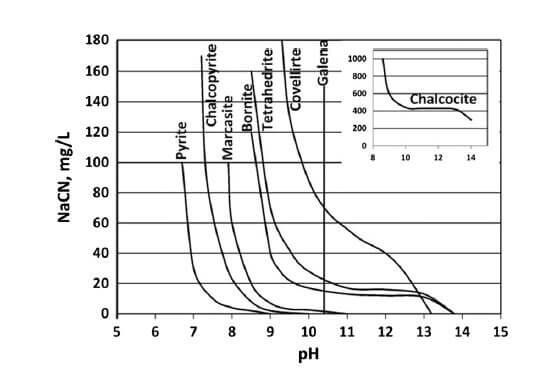
Cyanide Concentration and pH to Depress Sulphides
The chart displays how the Cyanide Concentration (how much NaCN) is needed to effectively Depress Sulphides like Pyrite, Sphalerite, Chalcopyrite but not Galena at various pH. It is pH that affects/impact the required effective concentration of cyanide. The higher the pH, the less NaCN is needed as CaO/Lime also acts as a Sulphide depressant. The use of […]
