Gold & Silver Dissolution and Cyanide Concentration

By far the most important cyanides used in cyanidation are sodium cyanide and calcium cyanide. The latter product is sold in an impure form analyzing close to 50% NaCN equivalent. The former is sold in various grades from 85 to 98% NaCN. In comparing the dissolving effects of the cyanides of ammonium, sodium, potassium, magnesium, […]
Cyanide Leaching Chemistry & Gold Cyanidation
The reactions that take place during the dissolution of gold in cyanide solutions under normal conditions have been fairly definitely established. Most agree that the overall cyanide equation for leaching and cyanidation of gold is as follows: 4 Au + 8 NaCN + O2 + 2 H20 = 4 NaAu(CN)2 + 4 NaOH In a […]
Arsenic and Antimony Sulphide Minerals in Cyanidation

The successful cyanidation of gold ores containing appreciable amounts of arsenic or antimony sulphide minerals such as orpiment, realgar, and stibnite is usually difficult or even impossible. Extractions are low and the solutions soon become ‘foul.’ The gold in such ores could be free and uncoated, and no trouble in dissolving it in clean cyanide […]
How Copper Affects Cyanidation & Leaching
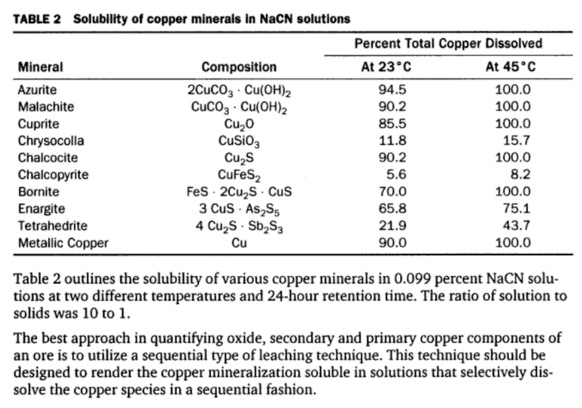
Many precious metal ores contain copper minerals in various amounts. These copper minerals dissolve in cyanide solutions to a greater or lesser degree depending on the particular copper mineral or minerals present, their fineness, and the dissolving effect of the cyanide solutions. In the process of dissolution the copper combines with, or as it is […]
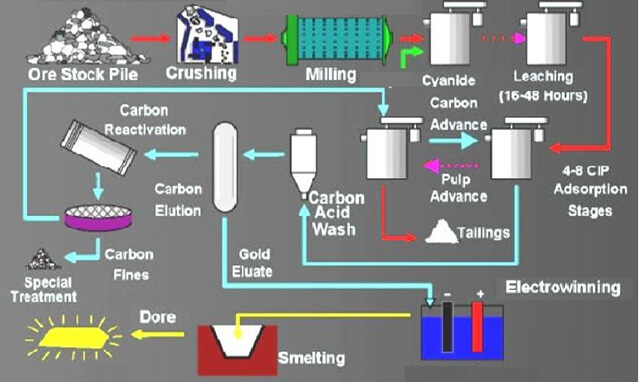
Process of Cyanide Gold Extraction

Here I present an Process EXAMPLE of Gold Extraction Cyanide in which the cyanidation feed consists of a pyrite concentrate floated after the selective flotation of a copper-gold concentrate. The pyrite concentrate is reground to 90% minus 325 mesh and aerated in a high-lime solution prior to cyanidation. It contains 99% sulphides of which 10 to […]
