Table of Contents
This report describes the preparation of tritium-labeled organic compounds to be used by the Federal Bureau of Mines in studying the role of certain gasoline constituents in gum-forming mechanisms. The gasoline constituents that have been investigated boil within a range of approximately 90 to 230° C. and can be classified as paraffins, naphthenes, olefins, diolefins, monocyclic aromatics, polycyclic aromatics, and organic sulfur, nitrogen, and oxygen compounds.
The technique of Wilzbach has been applied to more than 50 samples. Purification techniques have been developed that accomplish rapid and effective purification as well as removal of labile tritium in a one-step operation. Data are presented to show the rate of exchange between tritium and hydrogen that can be expected for a variety of compounds common to a full-boiling-range gasoline. These values should be useful to other investigators using tracer programs involving tritium labeled compounds.
Introduction
Development of liquid scintillation radioassay techniques for tritium during the past 10 years has made use of this isotope attractive for petroleum research. Wilzbach recently reported a simple and economical method of labeling organic molecules with tritium. The highly sensitive liquid scintillation counting techniques combined with the simplicity of Wilzbach’s exchange labeling provide a tool that is already widely used in biochemical research, and which undoubtedly will gain popularity in other fields as its versatility becomes better known.
Mechanism of Tritium Exchange
In the labeling technique described, the tritium has two separate functions. First, it is the radioactive label to be incorporated into the sample by exchange. Second, it serves as an internal source of radiation necessary to effect the exchange. Tritium gas ionizes by its own beta emission, producing atomic and molecular tritium ions and radicals. These interact with the organic molecules by reactions similar in mechanism to tritium recoil labelings by the He³(n,p)H³ and Li6(n,α)H³ processes. Investigators of these processes have observed both chain lengthening and chain degradation. In view of the chain lengthening observed in both recoil and exchange labeling, it may be concluded that these reactions are ion-molecule processes.
Three possibilities for primary steps in tritiation have been formulated by Ahrens and coworkers:
- T2 → T+ + He³ + beta
- Ionization of T2 or HT by beta radiation
- Ionization of organic compound by beta radiation
Many investigators have reported variation in specific activity for certain hydrogen positions in tritium labeled molecules. Rowland and others suggest that factors affecting this variation are probably: (1) Variations in steric shielding of the hydrogen. If the energy of the ionizing particle has been reduced to a few electron volts or less, the energy to penetrate a steric barrier may be quite important, making less likely the replacement of such shielded hydrogen atoms. (2) Structural isomerization occurring during the short time interval between the formation of the organic radical and the reaction of tritium with it to form a stable molecule. The probability of such isomerization would be expected to depend on which hydrogen was missing.
If the organic compound contains olefinic double bonds, the reaction can be one of addition to the double bond (saturation) rather than the substitution of tritium for hydrogen. Nystrom and Sunko report that molecules of the type R-C=C-Y, where R is an alkyl radical and Y is an ester radical, generally react with tritium by addition to the double bond. They found 100 percent of the activity of such compounds added to the double bond and 0 percent attached to the molecule by hydrogen substitution.
Apparatus and Reagents
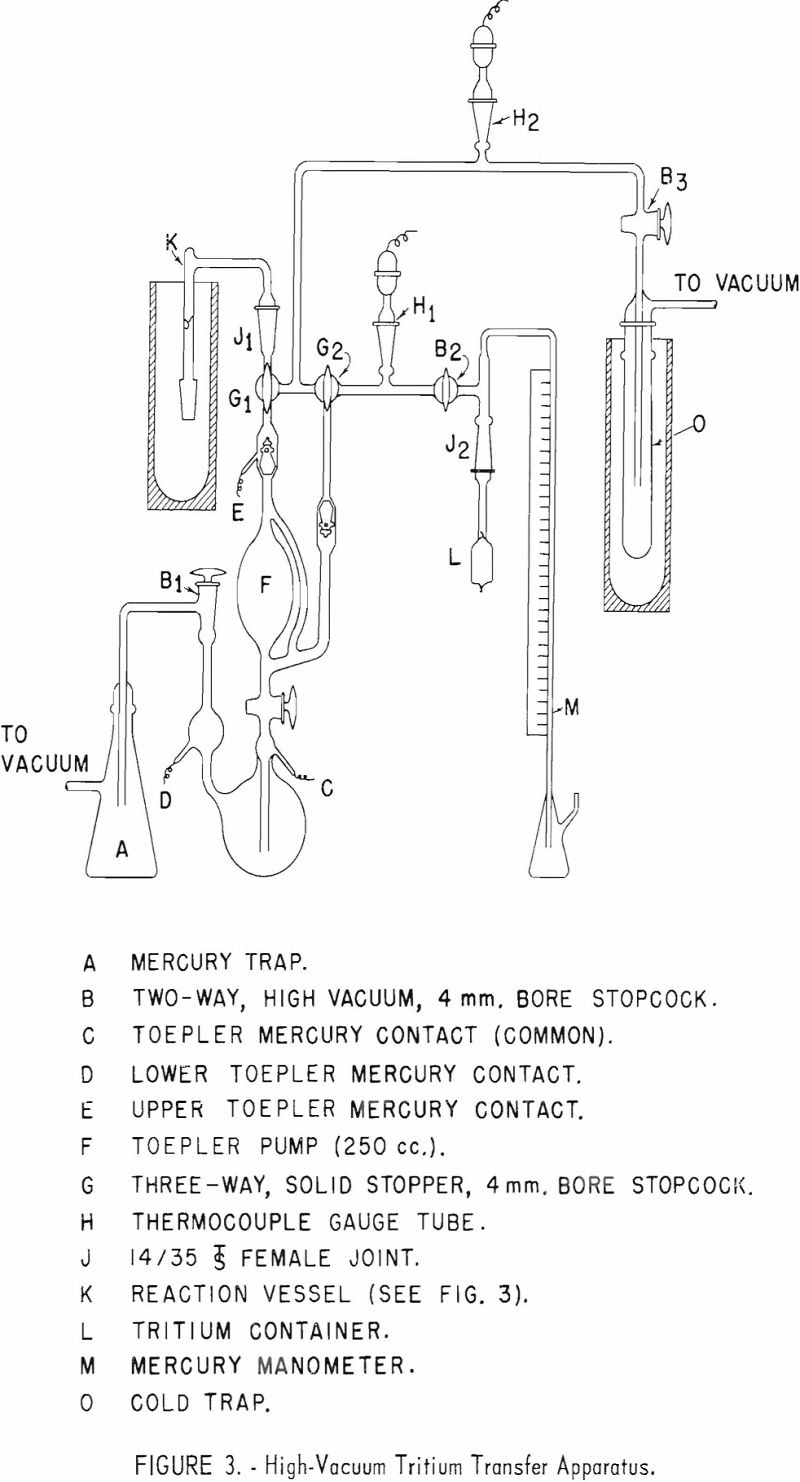
Appendix 1 presents details of equipment and operation of the apparatus used for transferring tritium gas into a reaction vessel containing an organic sample to be tagged with tritium.
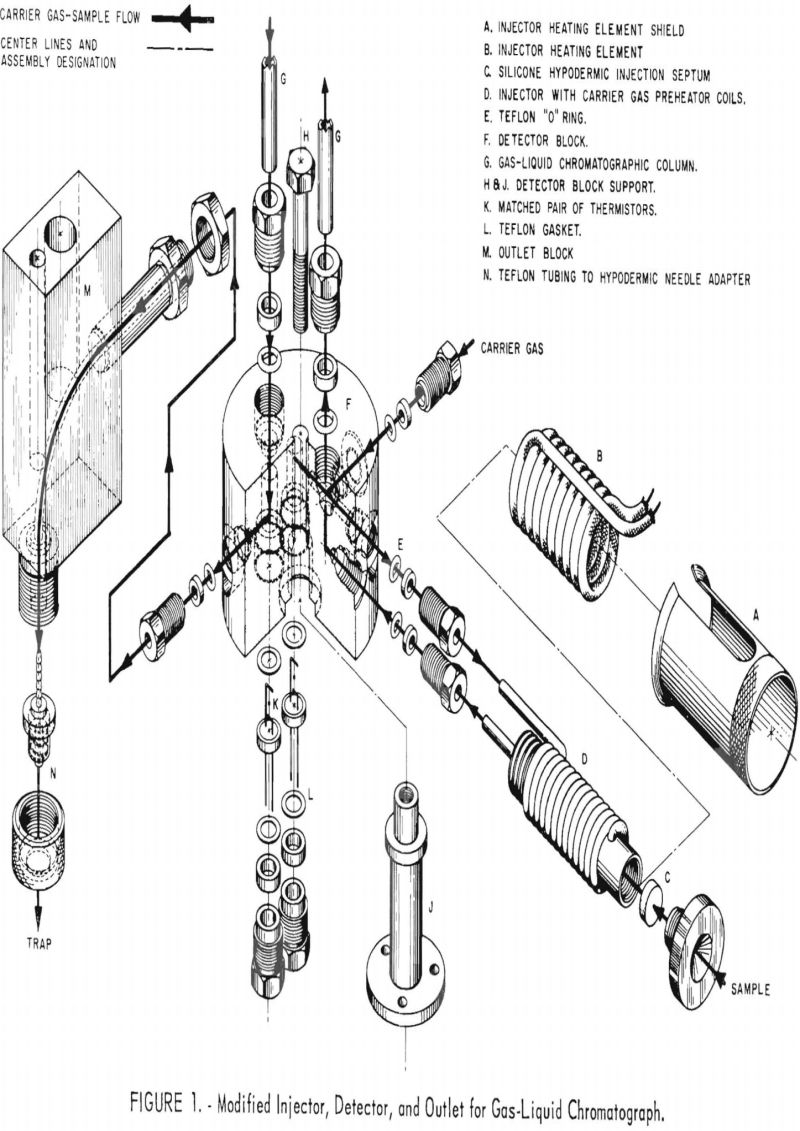
A modified Ferkin-Elmer gas liquid chromatograph (Model 154C) was used to purify tritiated materials. The sample injector, thermal conductivity detector, and heated outlet block were replaced with components designed to minimize the area exposed to radioactive contamination and to simplify decontamination following purification of high specific activity, tritium labeled, organic samples. Figure 1 shows an exploded view of the injection system, detector block, and heated outlet block as modified. Heat was provided to the injection system by a Thermacord heater distributed by Arthur S. LaPine & Co., Chicago, III. The detector uses a pair of matched thermistors (Model G112) supplied by Fenwal Electronics, Inc., Framingham, Mass. The sample transporting tubing between detector and outlet is standard teflon tubing. The outer block is heated by a 60-watt cartridge type heater.
A Packard Tri-Carb liquid scintillation spectrometer (Model 314) was used to radioassay all samples reported.
Tritium gas was supplied by Union Carbide Nuclear Co., Oak Ridge, Tenn., in small glass containers with a glass breakseal. It is necessary to fuse an appropriate size standard taper joint to this container so that it may be placed in position in the transfer apparatus. Appendix II describes the procedure used by this laboratory for safely fusing a standard taper joint to a vial of tritium gas.
Procedure
Labeling Organic Samples with Tritium
About 2.5 curies of tritium gas is transferred into a reaction vessel containing 1 gram of the sample to be tritiated as described in Appendix I. The vessel containing tritium gas and the organic compound is stored in a radio-chemical fume hood at room temperature for several days. The author’s experience is that 21 to 36 days are necessary to attain an activity level high enough that the labeled compounds are useful in studies of gasoline gum reactants. About twice weekly the reaction vessel is shaken to expose a fresh surface of the compound to the gas. Samples that are solid at room temperature are melted by a heat gun and are allowed to solidify over the entire inner surface of the reaction vessel.
Purification and Trapping
Even though pure materials are used for labeling, the ionizing energy of the beta particles produced by tritium decay produces radiation degradation products that usually have a high level of radioactivity. These byproducts must be removed before the parent material can be used as a tracer. Further, organic molecules containing groups such as the hydroxy, hydroperoxy, thiol, and amine groups contain active hydrogen atoms. Tritium that exchanges with these active hydrogen atoms is termed labile. This implies that the tritium is loosely bound to the molecule and will migrate readily to a similar position on another molecule under some conditions. Therefore, this labile activity must be removed from the molecule before the compound can be considered a valid tracer for most studies. Dissolving the sample that contains labile tritium in a hydroxylic solvent such as methanol will distribute the isotopes of hydrogen statistically between hydroxyl groups of the alcohol and active hydrogen positions of the organic compound. If large molar ratios of methanol to organic compound are used, the tritium may be effectively exchanged from a labile site in the tracer to the alcohol. When the hydroxylic solvent is removed from the system and fresh solvent is added complete removal of tritium from labile sites in the parent molecule can be approached. A simpler and more effective method for this operation was developed by the authors. The improved method employs gas-liquid chromatography (GLC) to purify the compound and remove labile tritium simultaneously by using a GLC liquid substrate containing several hydroxy functional groups, such as monohydroxyethyltrihydroxypropylethylenediamine. The vaporized tritiated molecule exchanges labile tritium for hydrogen furnished by the hydroxy groups of the liquid substrate as the sample passes through the GLC column. This technique has proved successful for many samples.
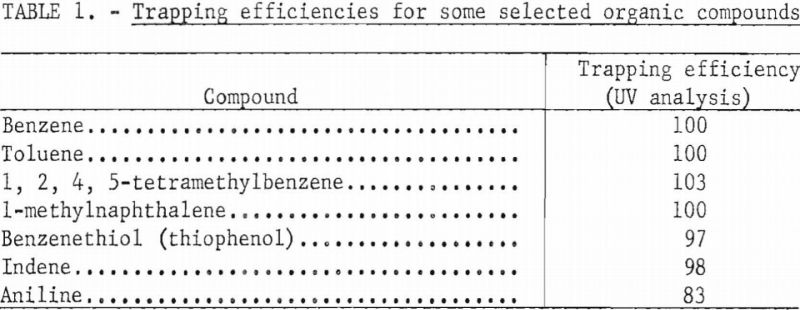
Purification techniques using GLC are fairly well established in the literature. Trapping techniques are perhaps less well established. Liquid bubble-type traps are efficient for recovering separated components from a GLC column. Using aromatic compounds and ultraviolet absorption analysis, Bureau investigators have established that trapping efficiencies of more than 97 percent can be obtained with bubble traps. Table 1 shows data obtained from trapping efficiency studies using this technique. Efficiencies were determined by ultraviolet analysis of aromatic samples trapped in isooctane. Low efficiencies were noted in tests with organic molecules containing nitrogen. This may have resulted from adsorption inside the GLC unit or low solubility of these compounds in the trapping solvent.
The bubble trap technique used by the Bureau involves directing the effluent helium-hydrocarbon stream from the GLC heated outlet block into 10 milliliters of an appropriate hydrocarbon solvent by means of a 17-gage hypodermic needle.
Some high molecular weight compounds may condense in the needle but may be quantitatively recovered by passing some of the hydrocarbon trapping solvent through the needle.
Decontamination of Equipment
Decontamination of the GLC equipment is necessary after the purification of each tritiated compound. The residual activity is not necessarily a health hazard, but is a source of radioactive contamination in later use of the equipment. Decontamination is facilitated by constructing columns from ¼-inch aluminum tubing and using each column only once for radioactive samples. The injection system and detector block are dismantled for cleaning. Teflon tubing which comes in contact with radioactive material is discarded. Thermistors are cleaned by immersing in aniline, alcohol, and benzene successively.
The dismantled injector and detector can be decontaminated most effectively and rapidly by immersing in Wedac decontamination detergent, supplied by West Chemical Products, Inc., Long Island, N, Y., in an ultrasonic generator for 30 to 60 minutes. This is followed by a similar ultrasonic cleaning in water for 10 minutes. A mixture of aniline-pyridine and acetone is then used in the ultrasonic generator tank for 30 minutes, and finally the parts are cleaned in acetone for 10 minutes, again using the generator. Although this procedure may seem long and tedious, it accomplished decontamination of the equipment more rapidly than other techniques.
Radioassay of Samples
The activities of all samples were measured with a liquid scintillation spectrometer using the internal standard technique reported by Whisman, Eccleston, and Armstrong. The scintillator solvent was toluene, the phosphor was 2,5-diphenyloxazole (PPO) and the wavelength shifter 1,4-di[2-(5-phenyloxazoyl)]benzene (POPOP). The precision of this technique of radioassay is about ± 2 percent and the accuracy is about ± 10 percent. The accuracy is governed by the accuracy of the internal standard radioassay, furnished by a commercial concern.
Results
Different types of organic compounds differ in susceptibility tritium exchange. Table 2 lists data for various compounds and blends of compounds that were labeled by the Wilzbach exchange method. The table includes the amount of tritium gas transferred into each vessel, the number of days of exchange at room temperature, and the exposure time expressed as curie-days. About one gram of sample was used in each of these exchanges.
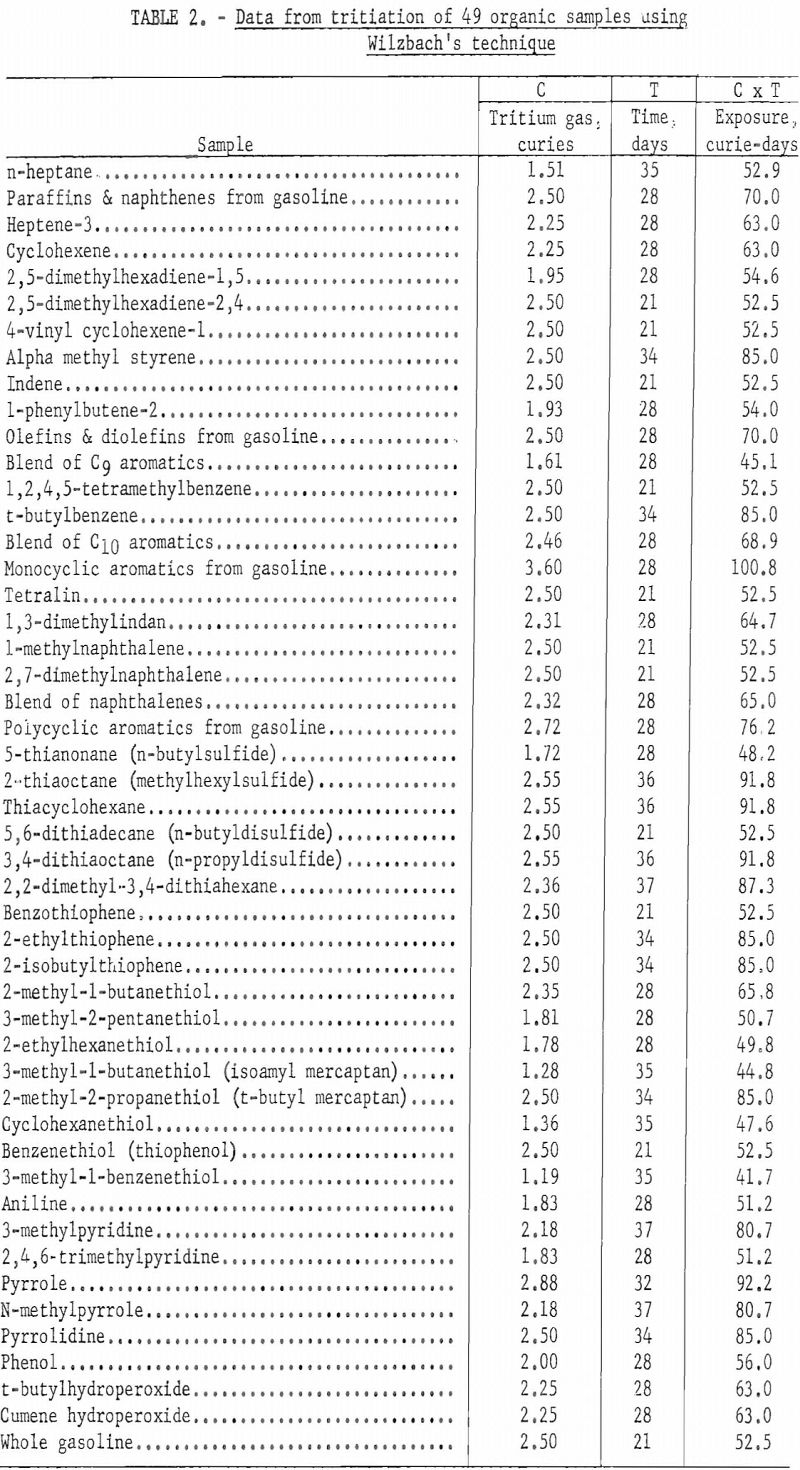
Table 3 lists the specific activities of the compounds listed in table 2 before and after purification. The values before purification include radioactivity associated with degradation products, as well as dissolved and labile tritium. A comparison of the specific activities before purification with the values after purification permits calculation of the percent of incorporated tritium that is in the parent compound as a usable tracer.
Because all samples were not exposed to the same amount of tritium gas and exposure periods varied from 3 to 6 weeks, some common denominator must be used to convert specific activities before and after purification to a comparable basis. For this purpose, curie days of exposure were used. Columns 4 and 5 of table 3 are calculated values based upon the specific activity in millicuries per gram of each sample and the exposure time in curie days.
The largest amount of tritium incorporation per gram of sample per curie day of exposure was achieved with compounds having olefinic unsaturation; relatively small incorporation was noted for paraffins, naphthenes, and thiols.
Although total tritium incorporation is significant a more important factor is the amount of tritium incorporated into the parent molecule being tagged. Column 5 of table 3 lists the tritium activity incorporated per gram of sample per curie day of exposure after degradation products and labile tritium were removed. These data are incomplete, because in many instances the compounds have not been or could not be purified. Although olefins had a high total incorporation of tritium, very little of this activity was usable as a tracer. Nystrom and colleagues reported that unsaturated straight chain compounds add tritium to the double bond, leaving a saturated compound. They report further that unsaturated compounds such as cyclohexene add tritium to the molecule in a ratio of about 84 percent by saturation of the double bond and 16 percent by substitution of tritium for hydrogen. Table 3 includes only one compound, heptene-3, that falls into the category of a straight chain unsaturated compound. This compound had about 7 percent of useful radioactivity or exchange of tritium by substitution. A terminally bonded olefin such as heptene-1 probably would follow the trend reported by Nystrom and yield very little, if any, useful radioactivity in the original molecule. The two cyclo-olefins listed in table 3 show less tritium incorporated by substitution than Nystrom indicated might be expected. The average such incorporation for these compounds was about 1.5 percent, compared to Nystrom’s value of 16 percent, For the two diolefins, apparently 7.9 and 1.7 percent, respectively of the tritium incorporated was by substitution. However, these values may be in error because of the purification techniques employed. It is difficult to separate monoolefins from diolefins of identical carbon number, using gas-liquid chromatographic techniques. It is conceivable that one double bond of some of the diolefin molecules was saturated by addition of tritium, leaving a monoolefin that was not separated by the purification.
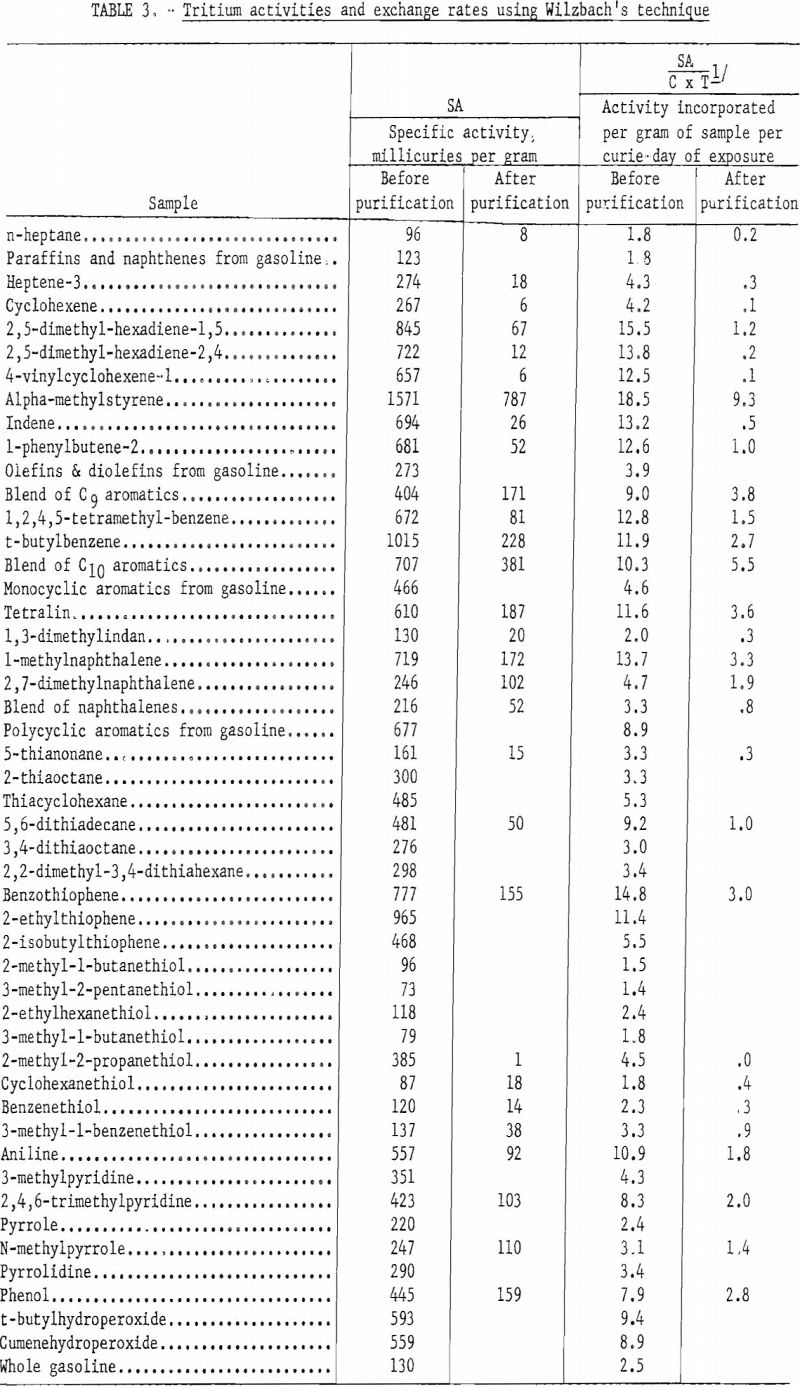
The only paraffin listed in this table is n-heptane, for which 11 percent of the total tritium incorporated was associated with the original molecule. Organic nitrogen and oxygen compounds and aromatic hydrocarbons gave average yields of about 30 percent. Aromatic olefins differed a great deal from simple olefins in their behavior toward tritium exchange. They more closely resemble the aromatic group in tritium incorporation than they do the olefin group. This would imply a preferential exchange of hydrogen for tritium by the aromatic ring.
Organic thiols appear to be extremely resistant to tritium incorporation in the molecule, and although about 20 percent of the total activity is associated with the original molecule, the tritium apparently is exchanged preferentially with the hydrogen associated with the sulfur atom. Because such hydrogen is active, (4) the tritium in this position is considered labile (loosely bound) and must be removed before the tracer is satisfactory for tests such as those described in this paper. Stripping tritium from this position left most tritiated thiols virtually free of radioactivity.
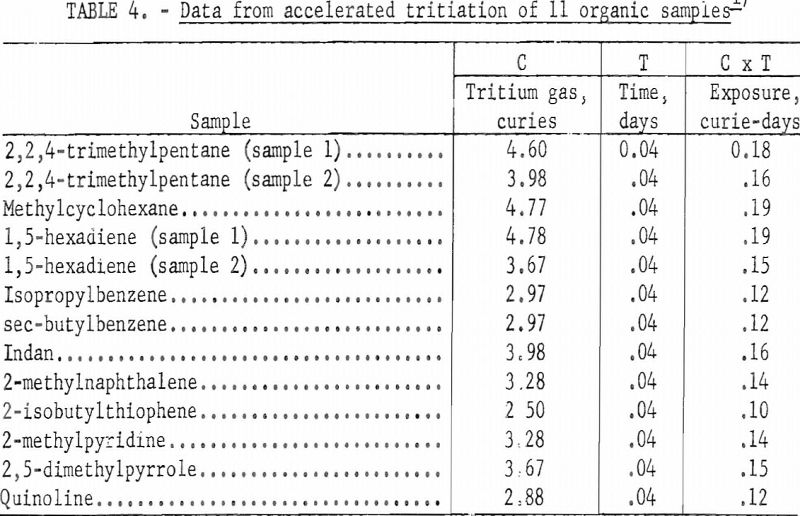
Table 4 lists data for 13 organic samples for which exchange of tritium and hydrogen was accelerated using an electric discharge. The apparatus used for this study was similar in design to that described by Lemmon and is shown in figure 2. A secondary energy source of 7-15 kilovolts at about 3 milliamperes was passed through the sample container for 1 hour.
Table 5 gives specific activities and activity incorporation for those samples accelerated by the electric discharge. The data have been treated similarly to that in table 3 for comparison. It might have been more realistic to tabulate the total tritium incorporation per curie-hour, but it would have been more difficult to compare with data in table 3. The difference in activity of duplicate samples in this table shows the effect of changing the electrical potential across the irradiation vessel. Sample 1 in each case was accelerated for one hour using about 7 kilovolts; sample 2 was accelerated for the same period of time, using about 15 kilovolts across the cell.
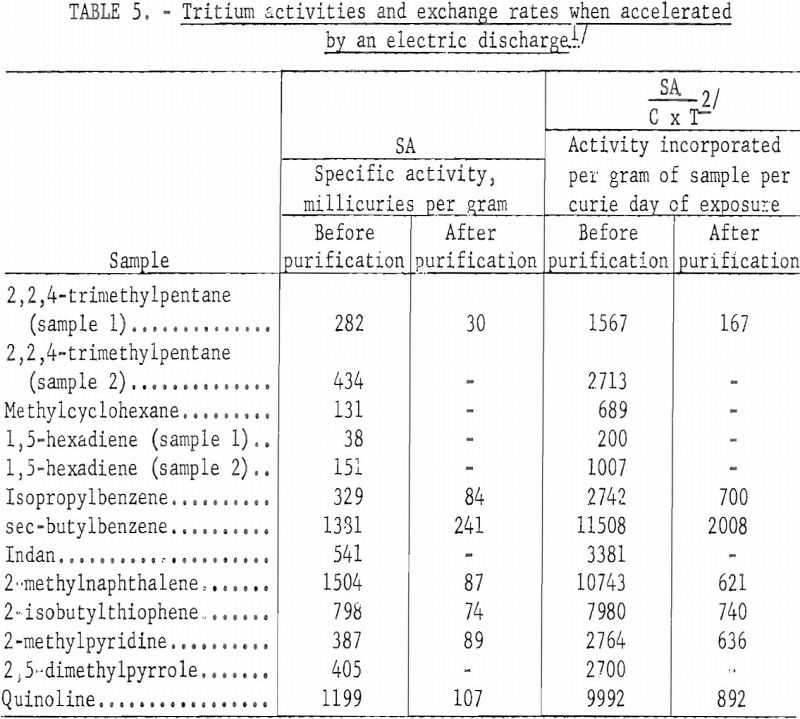
In the accelerated method the different classes of organic compounds showed about the same relative order in resisting exchange as in the Wilzbach method. About 10 percent of the total activity incorporated into the paraffin sample was associated with the original molecule and useful as a tracer. This is comparable to the data in table 3 using Wilzbach’s technique. However, the organic sulfur and nitrogen compounds and the aromatic hydrocarbons labeled by the electric discharge yielded only about 15 percent of the total activity incorporated as a usable tracer. This is approximately half the value obtained by the Wilzbach technique., and indicated a greater amount of activity associated with the degradation products using the accelerated technique. The presence of polymerization products in some of the reaction vessels gave visible evidence of excessive damage to the sample as a result of the electric discharge. However, in view of the simple and efficient means of purification for most organic products using GLC, most of the future tritium labeling work of this Center will be done by the accelerated technique despite increased damage products.
Conclusions
The data presented justify the following conclusions regarding tritium exchange labeling of organic, compounds.
- Tritium exchange labeling affords a convenient and economical method of preparing many radioactive organic compounds of the types found in petroleum. The rate of exchange can be accelerated by using the electric discharge method. However, excess degradation products by this method may make it unattractive for samples that are difficult to purify by gas-liquid chromatography.
- The techniques described are not satisfactory for labeling most aliphatic olefins or simple chain thiols because of the mechanism of labeling and the position of the label.
- Gas-liquid chromatography using certain column packing materials offers an efficient and rapid method of purifying the labeled compound and removing labile tritium.
- Petroleum constituents, subjected to the techniques described, varied in their susceptibility to tritium labeling.