Table of Contents
Many gold-bearing ores in the Western United States also contain small amounts of mercury. The presence of mercury creates potential pollution and health hazards during several steps in gold processing. An investigation was undertaken by the Bureau of Mines to develop techniques for removing mercury from cyanide mill solutions by selective electrowinning to reduce the potential hazards. Sixty-five pcrcent of the mercury was selectively electrowon from synthetic carbon strip solution at applied potentials of 1.00 to 1.50 V when using a mercury-coated platinum or copper plate cathode. Increasing the active surface area by using mercury-coated copper wool resulted in up to 96 pct of the mercury being electrowon from mill carbon strip solutions that contained 1.9 to 20 ppm Hg, 20 to 345 ppm Au, and 0.35 to 39.5 ppm Ag. About 98 pct of the silver was electrowon with the mercury onto the mercury cathode. Gold extraction ranged between 0 and 20 pct. Results of the investigation showed that choice of cathode material was critical to the success of the process. Selectivity was not achieved using uncoated steel wool cathodes.
A large number of low-grade gold and silver ore deposits are now being mined and milled throughout the Western United States. Total gold properties in Nevada alone number nearly 2,500, including 52 principal gold deposits. Almost 50 pct of the 1983 total U. S. gold production was from the approximately 550 million mt of reserves contained in these 52 principal deposits.
Cyanide leach liquors obtained from processing some of these deposits may contain gold, silver, mercury, and some base metals. The precious metals plus mercury and a portion of the base metals are recovered from the cyanide strip solution with activated carbon, then are eluted with a hot caustic cyanide solution. Gold, silver, and mercury are electrowon onto steel wool cathodes at applied potentials between 3 and 6 V. The loaded cathodes are removed from the electrolytic cell and retorted to remove the mercury, then smelted to produce dore bullion.
The mercury vapor present in gold refineries arises from the electrowinning, retorting, and carbon reactivation steps. These processing steps require high temperature, which causes the mercury to fume, creating a potential pollution hazard and a health threat to refining personnel. The additional processing steps that are required to maintain a safe environment in refineries add to processing costs as well.
The Bureau has previously investigated methods to precipitate mercury from gold cyanide solutions and slurries using sulfides. This technique, however, has limitations. Using silver sulfide as a precipitant ties up a considerable amount of silver, and excess precipitant would have to be recovered. Copper, iron, and zinc sulfides cause cyanide loss due to complex formation and also adsorb onto activated carbon. Calcium and sodium sulfides are effective in precipitating the mercury; however, some mercury sulfide redissolves with time.
An alternative method for removing mercury from aurocyanide mill solutions is electrolysis. This technique has two potential advantages over sulfide precipitation; (1) electrons are used to reduce the mercury, so no new chemicals are introduced into the solution, and (2) fewer processing steps are required.
Many metals, including cobalt, copper, gallium, gold, nickel, silver, tin, and zinc, can be recovered or refined by electrolysis in aqueous solution. Separation from multicomponent solutions, however, is difficult to achieve if the metals have similar deposition potentials.5 In practice, most electrowinning and electrorefining operations require high-purity electrolytes. Copper refining from acid sulfate solution, for example, requires elimination of bismuth from the electrolyte. Bismuth, if present even at a few thousandths of a percent, will cathodically precipitate with the copper and greatly reduce copper workability.
Deposition potentials of metals in solution can be altered by electrochemical and physical phenomena that result in polarization or deposition, and catalytic effects. Variables that may have a significant effect on deposition potential arc electrolyte composition, cathode material, and temperature. The interactions of these factors cause the plating behavior of a species to not be easily predicted, as the potential at which a metal actually deposits may be significantly different than its reversible potential. To illustrate, the deposition potential of most metals from complex cyanide solutions is known to begin at the reversible potential. But for others, copper and zinc for example, marked polarization occurs. Gold and mercury have similar reversible deposition potentials from aqueous cyanide solutions, but data in reference show that for these cyanide complexes there is a dependence of potential on temperature and cyanide concentration. Separation of bismuth and copper, which is not effective in acid sulfate solution, has been accomplished from a tartrate medium using copper and platinum cathodes.
Cathode material can have an extremely important effect on deposition potential. For example, the reversible potentials of copper and iron differ by 0.777 V. When a mercury cathode is used, the difference in deposition potentials is 1.30 V, which is a large enough difference to separate these metals electrolytically. Copper has been successfully electrowon onto a mercury cathode from solutions containing iron, and a flowsheet has been proposed for selectively electrowinning copper, nickel, and cobalt from acid sulfate or acid chloride solutions using a mercury cathode. These separations are possible using a mercury cathode because the over voltage of hydrogen on a mercury cathode is known to be greater than that of any metal. Over voltages are a function of the cathode used, and factors that affect over voltages also affect electrolytic reduction. A reduction process may occur at one cathode material with a much less negative cathodic potential than at another. It is therefore possible to extract metals from a dilute solution without generating hydrogen gas at a mercury cathode.
Temperature affects the conductivity of the electrolyte and therefore influences the required cell voltage. An increase of temperature increases:
- the rate of diffusion and convection,
- the effective metal ion concentration,
- rates of chemical solution at the anode and re-solution at the cathode,
- current efficiency, and
- solution conductivity.
It also stimulates crystal growth, as opposed to nuclei formation, decreases the current density and oxygen solubility, and promotes the evolution of hydrogen (22). The net effect is a decrease in polarization and a lower required cell voltage. The specific effect of temperature on gold electrowinning has not been extensively determined experimentally, but the prediction that a higher temperature would increase current efficiency was confirmed by Waterman.
Good electrolytic cell design is important to overall process efficiency and also influences the applied potential requirement for operation. Current efficiency is enhanced by using the lowest possible applied potential for metal reduction. Efficiency is also promoted by insuring good contact between the electrode and the electrolyte so the reacting species has ample opportunity to be reduced. A large cathodic surface area and good mixing are two methods that may be used to promote effective contact. Mixing also serves to reduce the thickness of the diffusion boundary layer, increasing the rates of diffusion-controlled reactions. Reducing or eliminating both solution and electrical bypassing of the cathode will also promote efficiency.
Based on these considerations, the Bureau initiated investigations with the goal of developing an electrolytic procedure for removing mercury from gold-containing cyanide solutions. The primary objectives were to develop an electrolytic cell suitable for laboratory tests and to determine the effects of cathode material, temperature, applied potential, and the presence of silver in solution on the efficiency of mercury winning and the separation of mercury from gold.
Laboratory Investigations
Solutions used in this investigation were synthetic gold or silver cyanide solutions and actual mill carbon strip solutions. Synthetic solution compositions were based on averages of several mill carbon strip solutions. Mill solution gold concentrations varied from approximately 20 to 400 ppm, mercury from 2 to 40 ppm, and silver from 0.5 to 400 ppm. Synthetic solutions contained a gold or silver concentration of 40 ppm and an equal concentration of mercury. Free cyanide and sodium hydroxide concentrations were 1 and 10 g/L, respectively. Gold, mercury, and silver were added from reagent-grade NaAu(CN)2, Hg(CN)2, and Ag(CN)2, respectively.
Electrolytic Cell Design
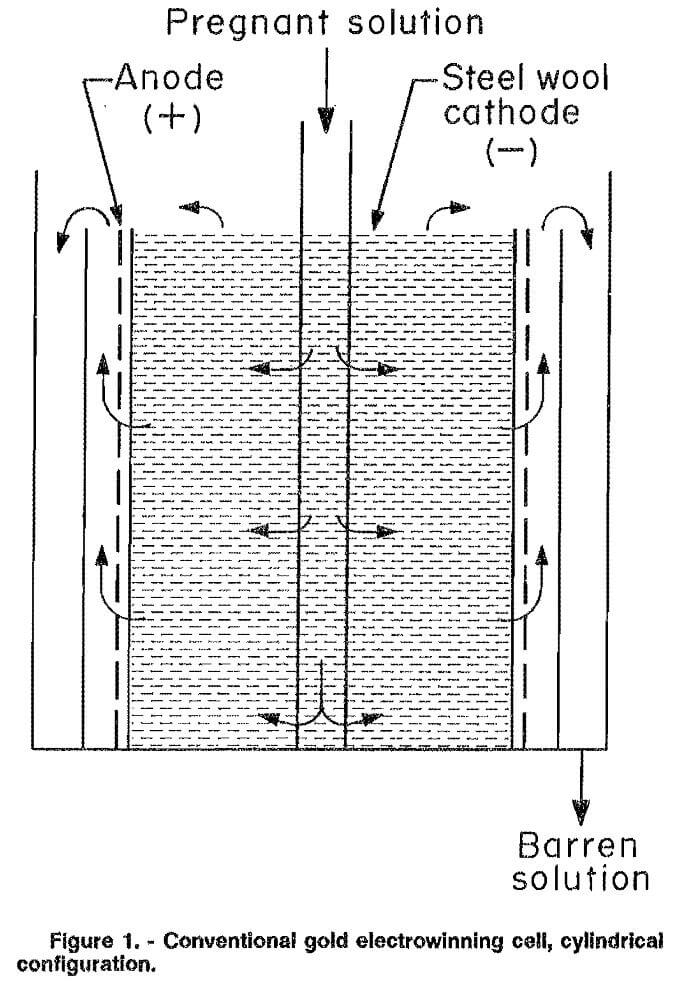
Two types of cells are commonly used for the electrowinning of gold and are classified according to their design as either cylindrical or rectangular. Both use porous packed-bed cathodes, usually of steel wool. The major difference between the cells is in the relative direction of solution and current flow. Uniform solution flow through the cathode bed is difficult to achieve in the cylindrical cell (fig. 1). The solution tends to flow to some extent along the axis of the cell perpendicular to the current flow, which is in the radial direction. In the rectangular cell (fig. 2), the solution and current flow are parallel to each other.
Inherent problems in cylindrical cells are mechanical and electrical bypassing of the cathode. Mechanical or solution bypassing occurs in these types of cells because it is very difficult to prevent the solution from flowing through the gap between the anode and cathode. As a result, the single-pass extraction efficiencies are usually low. Electrical bypassing is a result of excessive potential drop, which is the product of current and solution resistance, through the cathode bed. This potential drop may cause the magnitude of the electrode potential within the cathode bed to decrease significantly. If the decrease causes the electrode potential in a section of the bed to drop below the deposition potential, no metal plating will take place in that part of the cell. Flow of electrolyte through this inactive portion of the cathode bed is termed electrical bypassing. Electrical bypassing does not occur in cells where the electrolyte and current flow is parallel because the solution must flow across the entire cathode bed, thus contacting the active outer areas.
Figure 3 shows the electrowinning cell designed for use in this investigation. It is a cylindrical cell, but the current flow is parallel to the flow of electrolyte, as in a
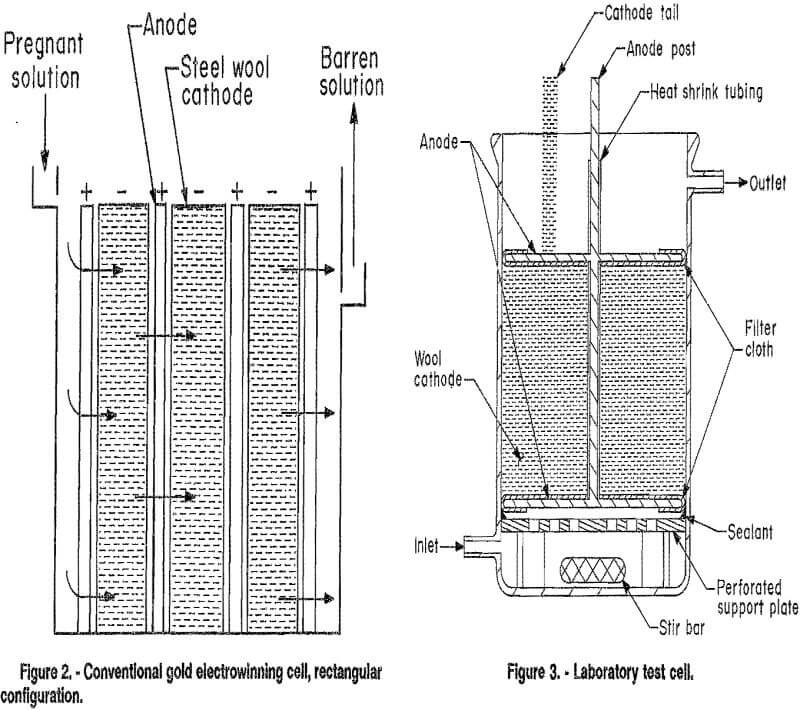
rectangular cell. Solution volume in the cell was approximately 350 mL. When steel or copper wool cathodes were employed, an electrode assembly was used that consisted of two stainless steel screen anodes centered on the ends of a stainless steel post that served as the electrical connector for the anodes and central support for the cathode. The electrode assembly was supported by a Nalgene polythylene platform set above the bottom of the cell to allow room for a magnetic stirring bar. The rim of the platform was sealed to the cell wall with RTV silicone adhesive. Central holes were drilled into the platform to force solution flow initially through the central portion of the cathode and to minimize solution flow along the cell wall. Solution bypassing of the cathode was further minimized by use of solution recirculation. Combined with mechanical agitation supplied by the magnetic stirring bar, recirculation provided good solution contact with the cathode and insured a thin diffusion boundary layer.
Two to four grams of wool cathode was wrapped around the central stainless steel anode post. The post was insulated from contacting the cathode by using heat shrink Teflon fluorocarbon polymer tubing. Electrical connection of the wool cathode was made through a “tail” of the wool, which was pulled up over the cell rim. Short circuiting between the anode faces and cathode was prevented by covering the side of the anodes nearest the cathode with polypropylene filter cloth.
Some tests were conducted using flat plate electrodes. For these tests, the electrode assembly was not used. The cathode was suspended vertically above the bottom of the cell, to allow room for a stir bar. The anode was a stainless steel plate suspended approximately 1 in from the cathode. Both faces of the cathode were active with a total active surface area of approximately 70 cm².
Test Procedure
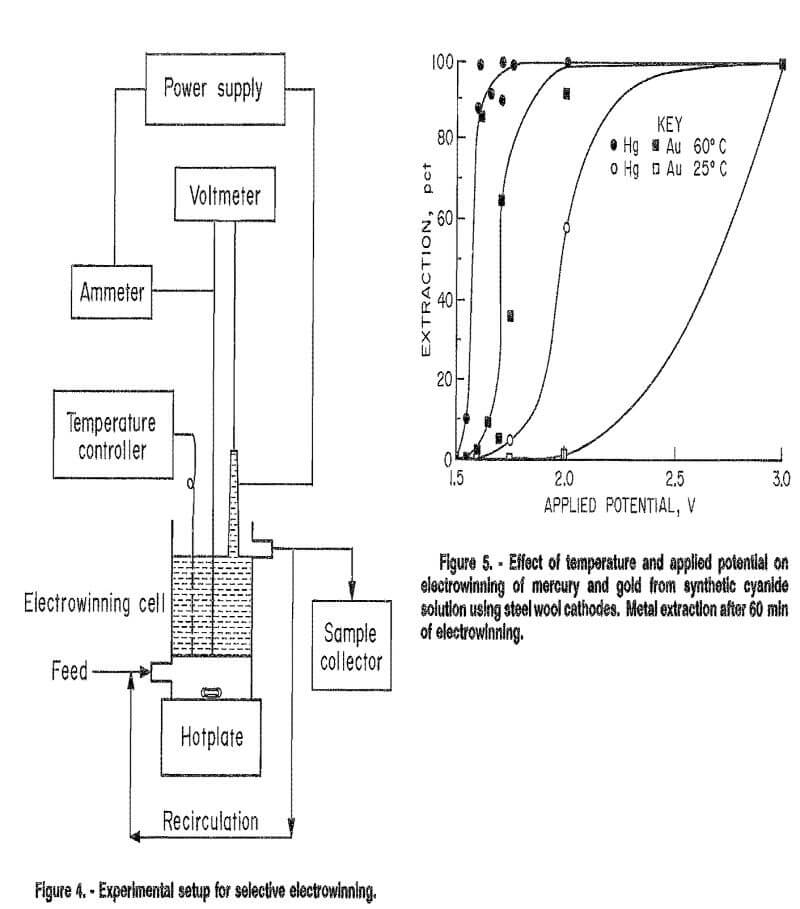
The experimental setup is shown in figure 4. Direct current was furnished by a Hewlett Packard model 6269B power supply. Current and applied potential were read by VIZ model WD 693 multimeters.
In all batch and continuous tests, the cell was operated in a multiple-pass mode, i.e., the electrolyte was continuously pumped from the overflow reservoir (in batch tests) or the cell outlet (in continuous tests), back into the cell inlet. The recirculation pump rate was 500 mL/min. Samples were taken at specified time intervals and analyzed for metal concentration by atomic absorption spectrophotometry.
For continuous tests, a feed pump was added to the system. Solution flow through the cell was maintained at 3 to 5 mL/min. This feed rate resulted in a retention time of approximately 100 min in the cell, which was the retention time required to achieve maximum extractions during batch tests. Continuous tests were conducted from 10 to 16 h. Depending on experimental conditions, steady state was achieved in 15 min to 4 h.
Tests Using Steel Wool Cathode
Temperature-Potential Tests Using Synthetic Gold Cyanide Solutions Containing Mercury
Synthetic Gold
Because steel wool is commonly used as the cathode material in gold electrowinning operations, it was initially
chosen as the cathode material. Two grams of 00 grade steel wool was cleaned for each test using a combination of washing with detergent, immersion in alcoholic sodium hydroxide and/or acetone to dissolve any oil or grease, and rinsing with deionized water. The first test series was made to determine the effects of applied potential and temperature on mercury and gold extraction. These tests were conducled in batch mode to obtain solution-residence time data for use in continuous tests. Tests were conducted at temperatures of 25° and 60° C under applied potentials ranging from 1.5 to 3.0 V. After a temperature-voltage test was completed, the cathode was either replaced, reused, or subjected to an anodic potential to dissolve any plated metal before the next test was run.
Results of temperature-potential tests using a steel wool cathode were inconsistent with each other. This is because the character of the cathode was not the same for all tests, nor during a single test. As mercury was won from solution, it coated the steel wool to some degree. Electrowon metal also remained on the wool from one test to another in some cases. However, general trends of the effects of temperature and applied potential were determined, and are summarized in figure 5. At 60° C, winning of mercury was successful at potentials above 1.50 V; gold winning was suppressed at potentials below 1.55 V. At 25° C, mercury extraction was slower and was suppressed below 1.70 V. Gold extraction was suppressed below 2.0 V. Mercury extraction was faster then gold extraction at both temperatures. Overall results of the batch tests indicated that selective electrowinning of mercury over gold may be possible at applied potentials below 2.0 V at 25° C, and below 1.55 V at 60° C. A series of continuous tests, described in the following section, produced different conclusions, however. These tests showed that effective separation of mercury from gold cyanide solutions may not be possible in a continuous process using a steel wool cathode.
Temperature-Potential Tests Using Synthetic Silver Cyanide Solutions Containing Mercury
Synthetic Silver
Tests were performed to determine the effect of silver in solution on the electrowinning of mercury. A comparison was made of extractions from gold solution containing mercury and from solution in which the gold was replaced by an equal concentration of silver. Results for the mercury-gold solution are taken from the temperature-potential tests discussed in the previous section. Test conditions for the mercury-silver solution were the same as those used for the temperature-potential tests using the mercury-gold solution. Results are shown in figure 6. Mercury extraction was faster at 3.0 V from the mercury-silver solution at both 25° and 60° C than from the mercury-gold solution. The rate of silver extraction paralleled the mercury extraction rate from the mercury-silver solution at both temperatures.
Results of temperature tests performed at an applied potential of 2.0 V are shown in figure 7. At both 25° and 60° C, mercury was extracted at approximately the same rate from both the mercury-gold and mercury-silver solutions, but extraction was depressed at 25° C. The silver extraction rate again paralleled the mercury extraction rate at both temperatures. The gold extraction rate at 60° C was similar to the silver extraction rate
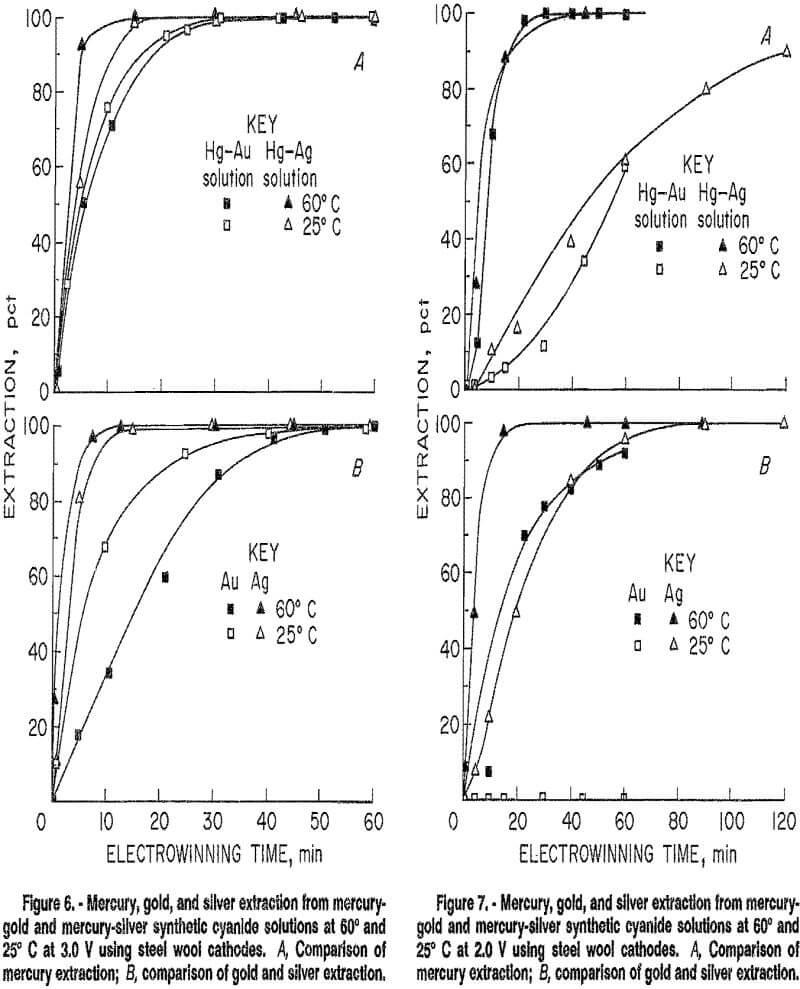
at 25° C. Gold extraction was completely suppressed at 25° C. Decreasing the temperature from 60° to 25° C at this potential had a much greater effect on extractions of all three metals than the temperature decrease at 3.0 V. Differences in electrowinning from mercury-gold and mercury-silver solutions were not positively determined by these tests, again due to the inconsistent, variable character of the cathode. However, they did indicate that silver might be won along with mercury from solutions containing both metals.
Tests Using Mercury-Coated Cathode Materials
Because a mercury coating on cathode materials is known to change the deposition potentials of some metals and accumulation of a mercury coating is unavoidable by the nature of the process, the remaining experiments were conducted using cathodes that were precoated with mercury.
Steel Wool
An attempt was made to coat the steel wool cathodes with mercury to determine if selective reduction and more consistent results could be achieved from cyanide solutions using this type of cathode. Coating was attempted by recirculating 400 to 500 mL of a synthetic solution, which contained 1 g/L free cyanide, 10 g/L sodium hydroxide, and 2 g/L mercury, at 25° C under an applied potential of 2.0 V. This was done until the current decrease stabilized, indicating a completion of the plating reaction. After the coating procedure for some tests, visual and microscopic inspection revealed that mercury had plated preferentially on some areas of the steel wool while other areas of the wool appeared uncoated.
The coating solution was drained from the cell and the cell was refilled with hot (60° C) synthetic carbon strip solution. The potential being tested was then applied, and after 5 min the feed solution pump was started.
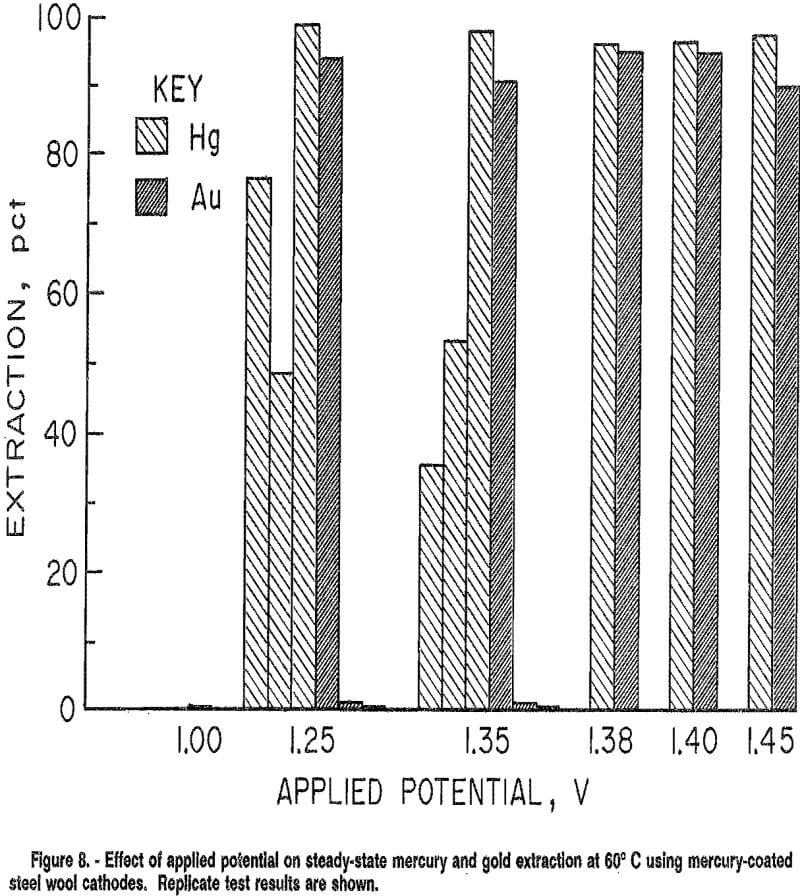
Continuous electrowinning tests were conducted at 60° C under applied potentials of 1,00 to 1.50 V for 12 h. Results are shown in figure 8.
Selectivity was realized at 1.25 and 1.35 V for some tests, with mercury selectively extracted over gold. However, reproducibility of results was difficult to achieve with this cathode. Replicate tests had markedly different results; for example, at 1.35 V, maximum extractions varied from 35 to over 99 pct for mercury, and from 0 to 94 pct for gold. Low gold extraction occurred with both high and low mercury extraction. These widely variable results may be explained by the difficulty in achieving a uniform adherent mercury coating on the steel wool.
A series of tests using the same conditions was made using steel wool coated with both mercury and gold to see if coextraction of gold had an effect on selectivity or reproducibility of results. The steel wool was coated using the same cleaning and coating procedures previously described. The synthetic electrowinning solution was used as the coating solution, and was recirculated through the cell at 25° C under an applied potential of 2.5 to 3.0 V until the current decrease stabilized. The test procedure established for continuous tests was then followed. Much better agreement among replicates was achieved, as shown in figure 9, although maximum mercury extractions at 1.35 V varied about 25 pct. Ninety percent of the mercury was selectively extracted at 1.25 V. A uniform gold-mercury coating was also difficult to reproduce.
Platinum Foil
To better control and reproduce the mercury coating, platinum foil was used for the coating substrate. Platinum has been used extensively in electroanalytical chemistry as the substrate for mercury thin film electrodes. Platinum does not amalgamate appreciably and therefore provides a good substrate to coat with a layer of mercury. The platinum cathode was cleaned first with detergent and rinsed with deionized water. It was then degreased in an alcoholic sodium hydroxide solution. After rinsing a second time in deionized water, the cathode was placed in boiling nitric acid. The cathode was then rinsed a third time in deionized water. If the final rinse water did not completely wet the electrode surface, the entire cleaning procedure was repeated. Further cleaning of the platinum cathode took place in the electrowinning cell prior to
coating the cathode with mercury. After filling the cell with the coating solution (1 g/L free cyanide, 10 g/L sodium hydroxide, and 2 g/L mercury), the electrodes were held under an applied potential of 0.0 V (anodic to hydrogen evolution and mercury plating) for 5 min, 3.00 V for 30 s to evolve hydrogen, then 0.0 V again for 5 min. The potential was then set at 2.00 V and the cathode was coated following the coating procedure used for the steel wool cathode. The test procedure established for continuous tests was then followed. Tests were performed continuously at 60° C under applied potentials of 1.00 to 1.50 V.
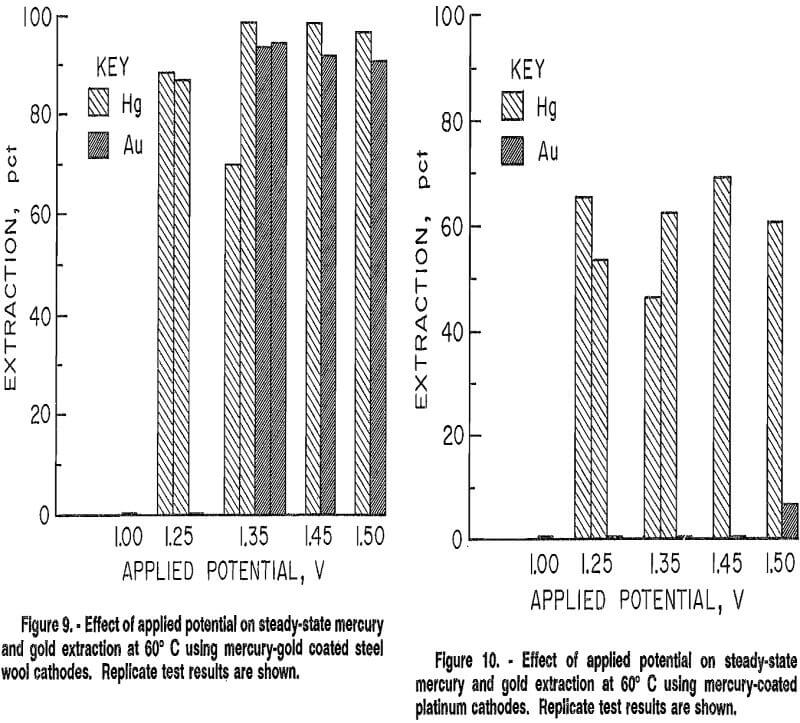
Results of tests conducted using this cathode (fig. 10) show that mercury was selectively won from gold at potentials between 1.00 and 1.50 V. Maximum extraction was limited to under 70 pct, possibly because of inadequate surface area. Replicate test results differed by 16 pet or less. These results indicated that an adherent solid mercury film coating increased reproducibility and selectivity over the irreproducible partial mercury or mercury and gold coating on steel wool.
Copper Plate
Tests were next conducted using a thin copper plate, of similar dimension to the platinum foil, as the substrate for the mercury coating. Because copper is a less costly material than platinum, it is better suited for industrial use. The cathode was cleaned in a manner similar to the platinum foil, except it was not placed in boiling nitric acid. Also, electrolytic cleaning was unsuccessful on the copper plate cathode because upon its immersion into the mercury solution, mercury began to plate onto the cathode. The copper cathode was found to be easily coated with an adherent mercury film following the coating procedure previously described. These tests were also conducted on a continuous basis at 60° C under applied potentials from 1.00 to 1.50 V. Figure 11 shows that mercury extraction was again limited to below 70 pct, and averaged 64 pct for all potentials from 1.00 to 1.50 V. Gold selectively remained in solution. Results are similar to those obtained with the mercury-coated platinum cathode, indicating that the effective cathode surface for both platinum and copper was mercury with no bare substrate exposed to the electrolyte to affect electrowinning.
Some copper did dissolve from the copper plate and was present in solution at steady-state concentrations, as shown in figure 12. The presence of copper in solution did not noticeably affect the electrowinning behavior of either mercury or gold, as results were comparable to results using the mercury-coated platinum cathode where no copper was present. These results indicate that to minimize dissolution of the copper cathode, electrowinning should be done using the highest applied potential for which gold is not extracted.
Copper Wool
The cathode area was increased in an effort to increase mercury extractions in continuous selective electrowinning tests. A cathode made of 99 pct fine copper wool was substituted for the flat plate copper cathode used in previous tests. The wool was cleaned with detergent and acetone before being placed in the cell. Four grams of copper wool was coated with mercury using the coating procedure described.
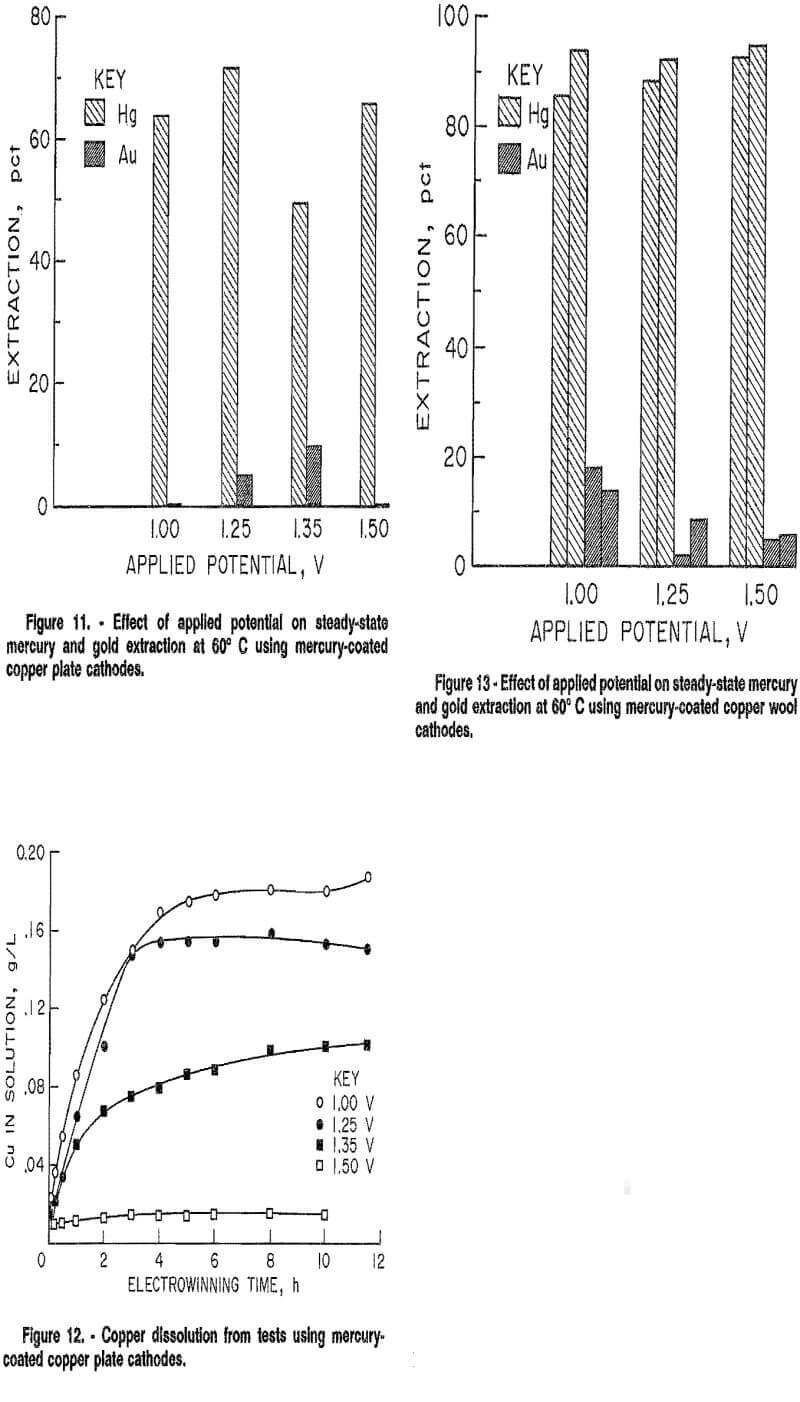
As shown in figure 13, selective electrowinning of mercury was successful for applied potentials from 1.00 to 1.50 V at 60° C. Ninety to ninety-five percent of the mercury was extracted for 12 h in all tests. Gold extraction ranged between 0 and 20 pct. The increased surface area of the copper wool increased mercury extraction an average of 27 pct over that achieved with the copper plate, with a slight overall increase in gold extraction of 5 pct. Reproducibility of results was within 5 pct.
The average steady-state current for these tests was less than 5 mA. Assuming a conservative surface area of the copper wool to be 900 cm², the average current density per test was 0,06 A/m².
Voltage efficiency is defined as the decomposition potential divided by the operating potential. The decomposition potential for mercury was found to be close to 1.00 V, since mercury did not deposit at lower potentials. Operating potentials were between 1.00 and 1.50 V, which resulted in voltage efficiencies of 67 to 100 pct.
Tests using Mill Solutions and Mercury-Coated Copper Wool Cathode
The effectiveness of the mercury-coated copper wool cathode was tested in continuous selective electrowinning tests at 60° C using two mill carbon strip solutions. The first solution contained, in parts per million, 20 Au, 20 Hg, 0.4 Ag, and 7 Cu. As shown in figure 14, selective electrowinning of mercury and silver from gold was successful at applied potentials of 1.25 and 1.50 V for this solution. Steady-state mercury extraction averaged 95 pct for these tests, while gold rejection averaged 98 pct. Between 50 and 75 pct of the silver was extracted with the mercury.
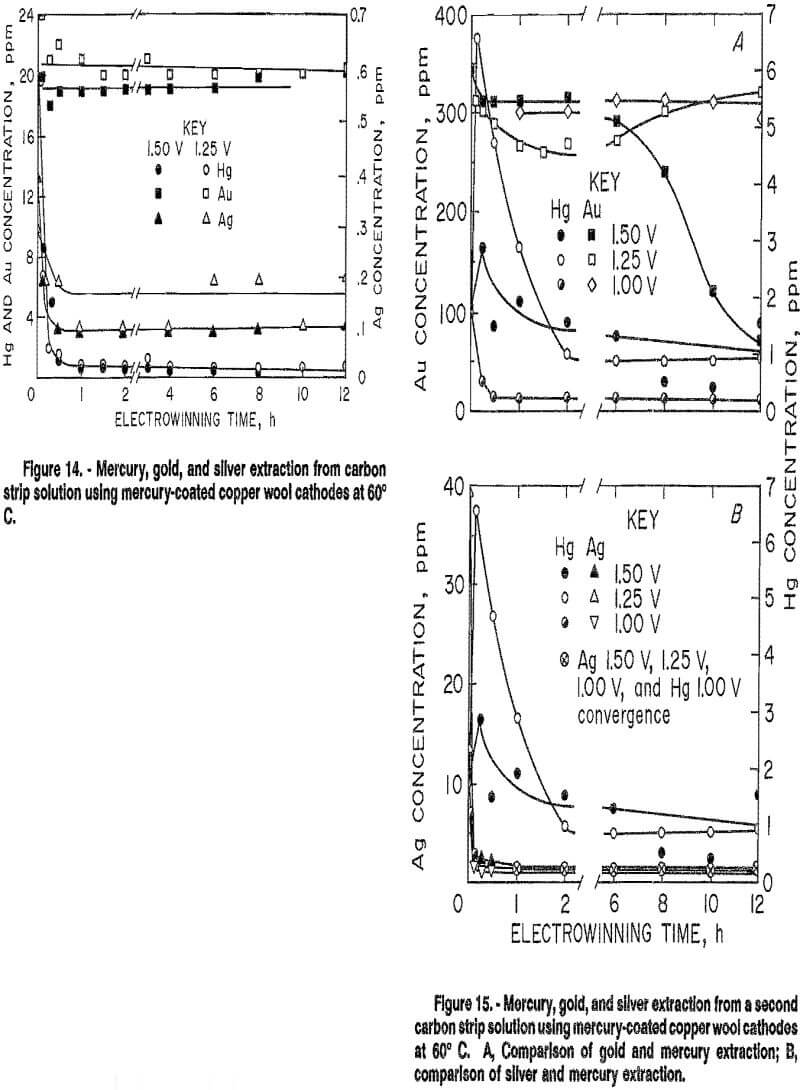
The second solution contained, in parts per million, 345 Au, 1.9 Hg, and 39.5 Ag. It also contained 157 ppm Fe and 944 ppm Ni. Results in figure 15 show that electrowinning at an applied potential of 1.50 V resulted in over 60 pct gold extraction, while mercury extraction was only 21 pct. Ninety-seven percent of the silver was also extracted. Decreasing the applied potential increased selectivity. At 1.25 V, gold extraction decreased to 19 pct, while mercury and silver extraction increased to 54 and 96 pct, respectively. A further decrease in applied voltage to 1.00 V resulted in extractions of 10, 90, and 98 pct for gold, mercury, and silver, respectively. A decrease in applied potential decreased the amount of mercury from the mercury-coated cathode going into solution at the initial stages of electrowinning. Increased selectivity in electrowinning mercury and silver from gold may be possible at potentials lower than 1.00 V for this solution.
Conclusions
- Selective electrowinning is a technique that is applicable in removing mercury from gold-containing cyanide solutions. Mercury was successfully won from synthetic and actual mill solutions containing a wide range of metal concentrations. Solutions used contained, in parts per million, 20 to 345 Au, 1.9 to 40 Hg, and 0 to 40 Ag, compared with mill solution assays, in parts per million, of 20 to 400 Au, 2 to 40 Hg, and 0.5 to 400 Ag. Silver tended to be won along with the mercury.
- The type of cathode used for selective electrowinning is critical to an effective process. Use of a material such as copper or platinum on which a uniform, adherent mercury coating can be applied improves the effective separation. In the tests described here, selective electrowinning was not successful using a steel wool cathode. However, steel wool would be a good cathode material to use for selective reduction if a method for achieving a uniform adherent mercury coating becomes available.
- Applied potentials required to selectively win mercury were much lower than those used in existing processes that win gold onto steel wool cathodes. Mercuiy was won from two carbon strip mill solutions at potentials of 1.0 to 1.5 V; extractions were, in percent, 90 to 95 Hg, 50 to 98 Ag, and less than 10 Au. By comparison, gold electrowinning is usually performed at 3 to 6 V.
- A solution temperature increase resulted in most effective operation. Required applied potentials were greater at 25° C than at 60° C.
