The economic removal of SO2 and production of elemental sulfur from stack gases discharged by base-metal smelters is a challenging-goal. Annually, in the United States, about 3.5 million tons of SO2 are discharged to the atmosphere by smelters. The SO2 concentration of the gases ranges from about 0.5 to more than 3 percent. As the sulfur content of SO2 is 50 percent, a potential production of 1,750,000 tons of sulfur per year is indicated.
The base-metal smelting industry in the United States now recovers part of the SO2, converting it to H2SO4. An outstanding example is the Kennecott smelter near Salt Lake City, where up to 80 percent of the SO2 is recovered as H2SO4. At many smelters, however, no SO2 or only a minor amount is recovered and converted to acid, primarily because of a lack of markets or because the gases are too dilute to permit economic production of sulfuric acid.
The removal of SO2 from a stack gas is not difficult. Scrubbing the gases with alkaline solutions, such as sodium carbonate, ammonia, or a slurry of lime, converts the SO2 to a mixture of sulfites and sulfates; but these solutions cannot easily be regenerated to recover sulfur or sulfuric acid. Their use in a once-through system represents a significant added cost of operation, and disposal of the spent-scrub liquors or slurries presents a severe waste disposal problem.
The Salt Lake City Metallurgy Research Center of the Bureau of Mines has been investigating an innovative process for removing SO2 from smelter gases that results in substantially simultaneous removal of SO2 and recovery of elemental sulfur. This paper describes the current status of research using simulated smelter gases containing 0.3 to 2 percent SO2.
Process Description
The proposed process for removing SO2 and producing sulfur from smelter gases is based upon the phenomena that when SO2 and H2S are absorbed in a suitable aqueous or organic liquid, they react rapidly, even at room temperature, to form elemental sulfur and water according to the stoichiometry shown in reaction 1.
SO2 + 2H2S → 3S + 2H2O………………………………………………(1)
Use of this technique has been patented for recovering sulfur from sour gas. In this application, the H2S-bearing gas is contacted with a liquid capable of absorbing H2S while simultaneously introducing SO2 produced by burning one-third of the recovered sulfur with air. The process has not been applied commercially, but claimed advantages over the conventional ethanolamine absorption and Claus-Chance Process for producing sulfur from sour gas are (1) a higher yield of sulfur using a lower grade H2S gas and (2) elimination of the absorption and desorption steps to separate H2S from methane or other gases. On this basis, an SO2 removal process encompassing the following simple unit operations was conceived and is now being studied on a laboratory scale. A simplified flowsheet is shown in figure 1.
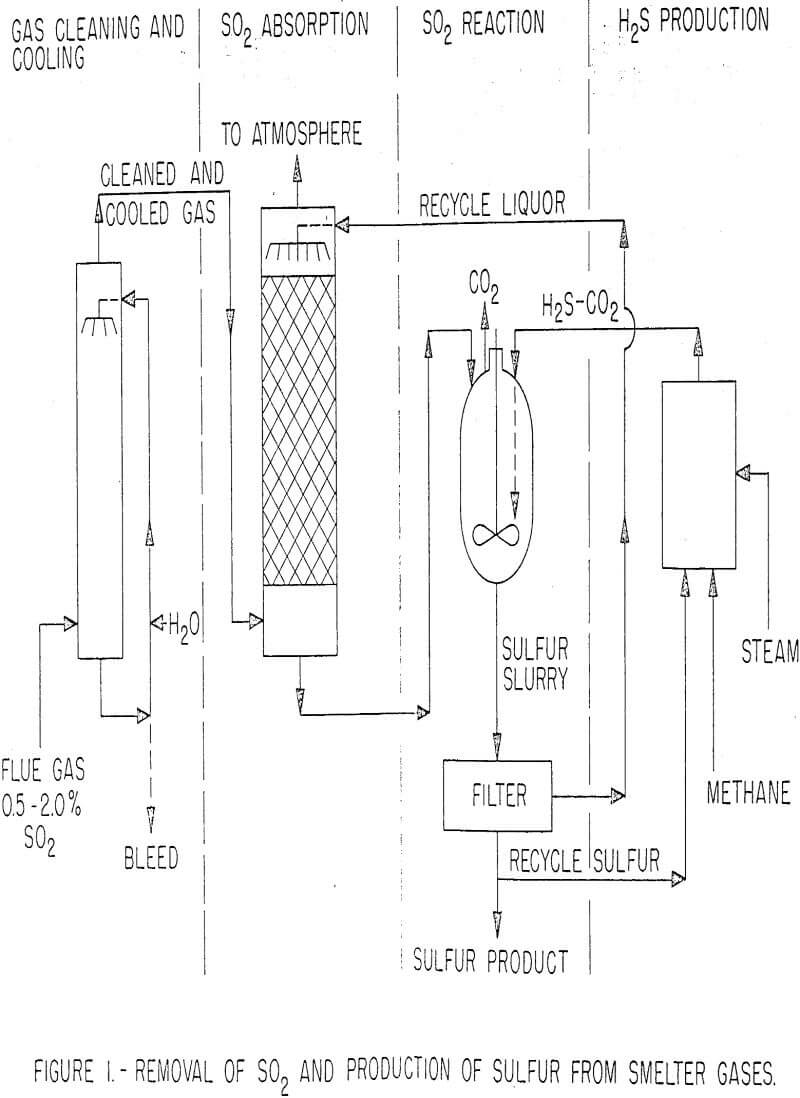
- The gas is cleaned of particulate matter and SO3 and cooled in a water spray tower complemented by an electrostatic precipitator, if necessary.
- The SO2 is absorbed in a packed tower by a suitable liquid.
- The SO2 loaded liquid is reacted with H2S, the sulfur formed is recovered, and the absorbent liquid is recycled.
- Two-thirds of the sulfur is recycled and reacted with methane and steam to produce H2S needed for the reaction.
Chemistry
Although the overall stoichiometry of the process involves the reaction of 1 mole of SO2 with 2 moles of H2S, the chemistry is much more complex. Chemical analyses of the recycled solutions show that they contain polythionic acids and polythionates ranging in sulfur content from 2 to 6 atoms per molecule, in addition to thiosulfate, sulfite, and sulfate. The chemistry of the process is still not fully understood, but a few of the more likely reactions with a sodium citrate absorbing solution, based on analytical studies and on published information, are as follows:
(NaO)3Ct + 3SO2 + 3H2O ↔ 3NaHSO3 + (HO)3Ct………………………………………(2)
3NaHSO3 + 6H2S + (HO)3Ct ↔ 9S + (NaO)3Ct + 9H2O………………………………(3)
2NaHSO3 + S ↔ Na2S2O3 + H2O + SO2…………………………………………………….(4)
3Na2S2O3 + 2(HO)3Ct ↔ 2(NaO)3Ct + 3S + 3SO2 + 3H2O………………………….(5)
H2S + 3SO2 ↔ H2S4O6…………………………………………………………………………….(6)
6Na2S2O3 + 9SO2 ↔ 4Na2S3O6 + Na2S4O6 + Na2S5O6…………………………….(7)
H2S4O6 + 5H2S ↔ 9s + 6H2O…………………………………………………………………..(8)
3Na2S3O6 ↔ 2Na2SO4 + 2SO2 + Na2S5O6………………………………………………..(9)
In reaction 2, the SO2 is absorbed by a sodium citrate solution, resulting in the formation of sodium bisulfite and citric acid and a decrease in the pH. The SO2-loaded solution is then pumped to the reactor where reaction 3 occurs. Here, reaction with H2S causes the precipitation of sulfur; the sodium citrate is regenerated, and the solution pH increases. Some bisulfite also reacts with sulfur as shown in reaction resulting in the production of thiosulfate. Sodium thiosulfate can then react with citric acid, or possibly with polythionic acid, as shown in reaction 5, to produce more sulfur and regenerate more sodium citrate. Reactions 6 and 7 show how polythionates and polythionic acids may be formed. Although reactions involving production of S2O6 (dithionate) are not shown, it is sometimes formed, along with sulfate, by oxidation of sulfites. Reaction 8 demonstrates the decomposition of tetrathionic acid by H2S. When this reaction is occurring, large quantities of sulfur are precipitated. Reaction 9 shows the decomposition of sodium trithionate to produce sulfate, SO2, and pentathionate. This reaction is undesirable and can be minimized by controlling the composition of the absorbent and the operating conditions.
Experience with a continuous aqueous absorption-reaction system has shown that after the initial formation of lower polythionates, reaction with SO2 leads to the formation of higher polythionates and a decrease in the pH; in contrast, H2S reacts to break down the higher polythionates to sulfur and water, accompanied by an increase in pH. Sulfur is also made when thiosulfate is decomposed by the polythionic-acids, so a highly complex interreaction of the sulfur compounds is taking place in the system. The reactions can be controlled to give adequate absorption of SO2 and at the same time produce sulfur, by the use of a solution that will buffer at a pH of 3.0 to 3.7.
Aqueous solutions that have worked well are sodium acetate and sodium citrate. Currently, the preferred solution is a sodium citrate solution containing sense excess citric acid and buffering in operation at a pH of 5.2 to 3.5. Sodium acetate solution is a superior SO2 absorbent, but the high vapor pressure of acetic acid (120° C boiling point) results in excessive losses. Citric acid decomposes above 175° C, but losses in tests at 50° C appear to be nominal. The capacity of a 1 M sodium citrate solution to extract SO2 from gas containing 0.5 to 2 percent SO2 ranges between 5 and 20 grams per liter at 30° to 60° C.
The chemical reactions which occur in a system using an organic absorbent are not as complex as those in the aqueous system or else the polythionic acids which form in reactions similar to reaction 6 are less stable and break down readily to form sulfur and water as shown in reaction 8. The currently preferred organics are tributyl phosphate (TBP) and tributoxy ethyl phosphate (TBEP). TBP and TBEP boil at nearly 300° C and have a vapor pressure of less than 0.03 mm at 50° to 60° C. Their SO2 capacity at these temperatures is 10 to 20 grams per liter when absorbing SO2 from a 0.5 to 2 percent SO2- air mixture. Other classes of organics of possible use include the glycols and modified silicones.
Experimental Results
Two small-scale SO2 absorption units have been operated to obtain data under continuous operating conditions. Most of the work has been done with the equipment shown in figure 2 using aqueous scrubbing solutions. The SO2 absorption column is a glass tube, 2 inches in diameter and 24 inches tall, and is filled with 3/8-inch Raschig rings, or more recently with 6-mm saddles. The reaction vessel where SO2 is reacted with H2S is of about 2 liters capacity and is equipped with a stirrer. Variable-speed-roller pumps are used to pump solution from the column to the reactor and to return the regenerated scrubbing liquid to the top of the SO2 absorption column. Air, SO2, H2S, and the flow of solution are metered through flowmeters.
After absorbing SO2 from the gas stream, the SO2-loaded solution leaves the bottom of the column and flows to a small liquid-level controlled hold tank. From here it is pumped to the reactor. After reaction with H2S, the sulfur-bearing slurry overflows to the filter funnel and the clear filtrate goes to a small hold tank from which it is pumped back to the top of the absorption column. The filter holds about 6 to 8 hours production of sulfur.
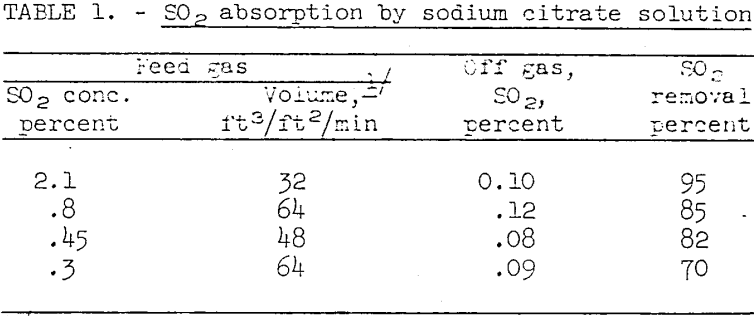
Table 1 shows the results obtained with an approximately 1 M citrate scrubbing solution when absorbing SO2 from SO2-air mixtures containing 0.3 to 2.1 percent SO2. The flow of solution through the scrubbing tower was held constant at 1.8 gallons per square foot per minute. The tests shown covered an operating time of about 40 hours. During this period the concentrations of thiosulfates and polythionates stabilized, indicating that SO2 and H2S were reacting substantially stoichiometrically to form elemental sulfur. The sulfur produced during these tests was a pale-yellow, high-purity, crystalline, and easily filtered product.
As indicated by the data given in table 1, the percent SO2 removed tended to decrease with decrease in SO2 content of the feed gas and with increase in volume of feed gas when using a constant flow of absorbing solution. As the absorbing solution was not saturated with SO2 the decrease in recovery merely indicates that the driving force of the reaction decreases with decrease in concentration of SO2 in the feed gas. More complete removal of SO2 at higher gas to solution flow rates presumably is achievable by using a taller absorption column.
A problem not reflected in the data given is the difficulty of washing the significant amounts of absorbed or entrained citrate out of the sulfur product. Preliminary wash-filtration tests have indicated that relatively large proportions of water are required to reduce citrate losses to less than 10 pounds per ton of sulfur produced. Such a loss is not exhorbitant, but the wash solutions are too dilute for direct reuse. Further work is needed to resolve this problem.
The unit shown in figure 3, which is 2.5 inches square by 36 inches long, has been used with TBP as the absorbing liquid. Trays composed of 1/8- by ½- by 2-¼-inch wooden slats are used for packing. Because losses of organic due to vaporization in the SO2 absorption column could represent a significant cost under commercial operating conditions, provision is made to heat and humidify the SO2-air mixture to approximate conditions at a smelter where the hot gases would first be scrubbed with water to cool them to 50° to 60° C. The column was designed to allow operation at higher gas flows than is possible in the packed column.
The main use of this system has been for determining TBP losses by vaporization when operating at 52° C (125° F). To determine such losses, heated and humidified air (9 percent) was passed through the column at a flow of 135 cubic feet per square foot per minute counter-current to a flow of TBP of 1.8 gallons per square foot per minute. The off-gas from the tower passed through a demister and knockout chamber, then through a cooling coil operated just above freezing to a condensate trap, and finally through an activated carbon filter. At the end of the test, the knockout trap, cooling coil, and condensate trap were flushed with kerosene, and the kerosene solution was analyzed for TBP using both chemical and infrared spectrophotometer techniques. The activated carbon was similarly analyzed. Results of duplicate tests showed a TBP loss of 4.6 grams per 1,000 cubic feet of gas. The measured loss was only one-third of the theoretical loss based upon the equilibrium vapor pressure of TBP at 52° C. The low measured loss is attributed to a lack of equilibrium at the high gas velocities.
Production of H2S
The chemistry of the proposed process requires recycling of two-thirds of the recovered sulfur to produce the H2S needed to reduce SO2 to sulfur. This may be most economically accomplished by reacting the sulfur with natural gas and steam to produce H2S and CO2. The reaction proceeds in two steps:
CH4 + 4S → 2H2S + CS2………………………………………………….(10)
CS2 + 2H2O → 2H2S + CO2………………………………………………(11)
We have not yet investigated this reaction, but the process has been studied by Texas Gulf Sulfur Co. They found that in the presence of an activated alumina catalyst, the first reaction proceeds substantially stoichiometrically at 600° to 700° C and gas residence times of less than 2 seconds. The second reaction is even more favorable. Practically complete conversion of CS2 to H2S and CO2 was achieved at 200° to 300° C in less than 0.5 second. The composition of the gas would be about 80 percent H2S and 20 percent CO2. Separation of the CO2 probably would not be required since batch tests with mixtures of H2S and CO2 have indicated that the reaction of SO2 in citrate solution with H2S is not adversely affected by the presence of CO2.
Discussion
Preliminary research has identified several organic and inorganic solutions that are effective absorbents of SO2 from simulated smelter gas containing 0.3 to 2 percent SO2. These SO2-loaded solutions are also compatible to the reaction of H2S and SO2 to precipitate a readily filtered elemental sulfur.
The research has not progressed sufficiently to allow making cost estimates for the overall process, but some data are available for estimating chemical costs. Losses of citrate appear to occur principally by absorption on sulfur; and if adequate recovery techniques can be developed, the losses might be in the range of 5 to 10 pounds or $1 to $2 per ton of sulfur. The loss of citrate would be independent of the SO2 concentration.
For a TBP absorption system, the indicated losses by vaporization are about 4.6 grams per 1,000 cubic feet of gas treated; and the losses per ton of sulfur would be inversely proportioned to the SO2 content of the gas being treated. For a 2 percent gas, the estimated losses would be 12 pounds or about $5 Per ton of sulfur. Therefore, use of TBP is being considered primarily in connection with the removal of SO2 from richer smelter gases and SO2 gases produced by roasting of pyrite.
For producing the H2S used in the process, the requirement is 11,200 cubic feet of methane and 1,125 pounds of steam per net ton of sulfur. Thus, for natural gas at 25 to 40 cents per thousand cubic feet, the cost would be $2.80 to $4.50. In addition, about 50 cents worth of steam is needed. These costs are independent of the SO2 concentration in the smelter gas. Total chemical costs, then, might be in the range of $5 to $10 per net ton of sulfur.
