Table of Contents
Pure silver is readily attacked by strong hot sulphuric acid of SG 1.815, or about 90 per cent, strength. The reaction which takes place may be represented as follows:
2Ag + 2H2 SO4 = Ag2 SO4 + 2H2O + SO2
Silver sulphate so produced is very soluble in the strong acid, when it is hot, as little as one-fourth of its weight of sulphuric acid being sufficient. As a matter of fact, on applying the acid the bisulphate of silver forms which melts at a comparatively low temperature.
2Ag + 3H2SO4 = 2Ag HSO4 + 2H2O + SO2
When the melted mass is cooled the bisulphate crystallises out. By pouring the solution out into water a copious white precipitate of the bisulphate forms. If, however, the solution is carefully diluted by means of steam to about a sp. gr. of 1.65 or 73 per cent, sulphuric acid, and allowed to cool, hard yellow crystals of anhydrous silver sulphate are precipitated. The same crystals may be obtained by allowing an aqueous solution, or one of dilute sulphuric acid, containing the bisulphate in solution to cool down; a deposit of yellow crystals of Ag2 SO4 will form.
Dr. Percy states that one part of silver sulphate requires for its solution.
180 parts of cold water
88 parts of boiling water.
180 parts of cold sulphuric acid, 10deg. B. (or sp. gr. 1.07, about 9 per cent.)
30 parts of boiling sulphuric acid 10deg. B. (or sp. gr. 1.07, about 9 per cent.)
20 parts of boiling sulphuric acid, 20deg. B. (or sp. gr. 1. 16, about 18 per cent.)
4 parts of cold sulphuric acid, 66B. (or sp. gr. 1.81, about 90 per cent.)
¼ part of boiling sulphuric acid, 66 (or sp, gr. 1.81, about 90 per cent.)
55 parts of boiling solution of sulphate of copper, 10deg. B.
45 parts of boiling solution of sulphate of copper, 20 deg. B.
35 parts of boiling acid of sulphate of copper, 10deg. B.
Additional figures will be submitted subsequently.
No information could be gained as to the relative amounts of metals necessary for parting with sulphuric acid. It is obvious that for successful parting the metal dissolved must form a compound easily soluble in the solvent, otherwise the insoluble sulphate or compound formed will coat the alloy, and prevent further action. Copper, iron and zinc form such insoluble salts, silver sulphate is readily soluble in strong sulphuric acid, and also sodium sulphate.
Parting Gold-Silver Alloy with Sulphuric Acid
Alloys of 10 grains of gold, assaying 9992, were taken, and alloyed with 10, 15, 20 grains of fine silver respectively. Each alloy was rolled out in the same manner as was adoped for the nitric acid parting; 20cc of strong sulphuric, 92 per cent., acid was used in each case, and the cornets were boiled for one hour. The resulting gold was much paler in colour than when nitric acid was used on similar alloys. The alloy containing equal quantities of gold and silver appeared to be such a pale yellow colour that it was not anticipated that anything more than a superficial action had gone on. The acid was poured off, and the cornets washed with distilled water, then dried and weighed. The results are as follow:
It is, therefore, clear that strong sulphuric will separate practically all the gold from an alloy of one of gold to one of silver, and that its action is much more complete than nitric acid on such an alloy.
Gold-Zinc Alloys
To determine how zinc, silver, gold alloy would part, those metals were melted together in the following proportions:
Gold 9975
Silver 983
Zinc 10,100
Owing to the energy with which zinc and gold combine a small portion was lost through spitting.
The alloy was washed with water, then heated with dilute sulphuric acid (1 in 10), hydrogen was evolved freely. Ten cc. of strong sulphuric were added, and the solution boiled for one hour. The acid was poured off, and distilled water placed on the brown button left. It immediately became white, owing to the precipitation of the silver in solution on the zinc.
Ag2 SO4 + Zn = 2Ag + Zn S04.
On adding more acid, and boiling, and then washing with water and dilute nitric acid the weight of the bead was 9750. On cupelling it weighed 9658. The button still contained silver. It is, therefore, not practicable to part such zinc, silver, gold, alloys with sulphuric acid, even although such alloys will part readily with nitric acid.
Gold, Silver, and Sodium
An alloy was then taken consisting of:
Gold 64.7
Silver 23.4
Sodium 11.9
This was boiled in dilute, then in strong sulphuric acid, gave gold 988 fine; this when fused with sodium bisulphate gave gold 993 fine. This shows that a very small amount of sodium is sufficient for parting with sulphuric acid. The alloy requires such a low temperature for melting, and parting can be done so easily, and there is no impurity introduced which cannot easily be eliminated. With sodium at a low price the method of parting could be advantageously practised in many cases.
Effect of Tellurium on Parting
This experiment was made to see if comparatively small quantities of tellurium had the same effect as sodium in enabling acid solutions to dissolve out silver from gold bullion. An alloy of gold and silver, containing 72.14 per cent, of gold, the balance being almost pure silver, was taken and melted with tellurium in a reducing flame.
Alloy 17.321
Tellurium 2.143
Total 19.464
After being gently warmed with sulphuric the characteristic crimson colour denoting the solution of tellurium appeared ; the alloy was boiled for one hour with strong sulphuric acid; it was then washed and dried. It weighed 19.114. The acid had, therefore, only dissolved a small portion of the silver and tellurium.
It was then treated with 1cc of sulphuric acid, and 10 grms. sodium bisulphate, and heated to dull redness; a violent action went on, and silver, as well as tellurium dissolved. After washing the soluble salts away the button was cut with a knife. The outside had been attacked, and the solvent had penetrated for about 1-16th of an inch, but the inside was lead grey and waxy. The button was broken into three pieces (a small amount was lost in so doing), and sulphuric acid again added, with a few crystals of sodium nitrate. A strong purple colour showed that tellurium was dissolving, five grammes of sodium bisulphate were subsequently added, and heat applied to redness. The slag was yellow hot and faintly greenish in cooling. On washing with water a voluminous precipitate of tellurous acid separated out. This was readily soluble in dilute sulphuric acid. The gold remaining was dried, and weighed 12.245grs. It was then parted in the usual way, and the parted gold weighed 12.005 grains. The fineness of the gold was, therefore, 98.04. This experiment shows that gold only 64.2 fine can be raised to nearly pure gold through the presence of about 10 per cent, of tellurium. If the alloy had been crushed to a fine powder, or even granulated, the action would have been more rapid. Solid masses containing only a relatively small amount of soluble material are difficult to decompose with solvent. The solvent has to find its way through the insoluble material, and if the spaces between the particles are small, the friction is very great; secondly the substance dissolved must diffuse in an opposite direction into the body of the solvent, and thirdly, the evolution of gas, which generally takes place from the particles attacked, has to escape, and thereby checks the inward flow of the solvent. The retardation of solvent action must, therefore, increase with the thickness of alloy to be penetrated.
Parting of Gold and Silver Commercially
Gold is not soluble in sulphuric acid except when nitric acid or some other oxidising agent is present. In this case it is again precipitated on dilution with water. Neither platinum nor palladium are dissolved when these are present in a silver alloy.
Copper is dissolved when alloyed with silver, but owing to the insolubility of copper sulphate in strong sulphuric acid the action is either retarded or prevented. Copper, also, owing to its low equivalent, consumes nearly three and a half times as much sulphuric acid as silver.
Zinc behaves in a similar manner to copper.
Iron is also slowly attacked, white iron, containing phosphorus, being less attacked than grey pig. The sulphate of iron formed is insoluble in strong sulphuric acid.
Tin and antimony are also dissolved, but form basic salts on dilution.
Lead is slowly attacked, if in large quantities, but in small it dissolves readily. If present in excess lead sulphate separates out from strong acid solution. On dilution with water the lead sulphate dissolved by the strong acid is thrown down.
From the foregoing facts in separating gold from silver it is advisable to have only the two metals present; small amounts of other metals do not interfere, but large amounts occasion trouble. For this reason base bullion is usually first treated by a process of liquation to eliminate the copper. If the base alloy be placed in a lead bath, heated strongly, the gold and silver will dissolve in the lead, while the copper, on cooling down, will form an alloy with the lead, and float on the top. It may then be stiffened with ashes or such material as will entangle it, and scraped off. The gold and silver can be obtained free from other metals by a process of scorification, or, as is termed on a large scale, cupellation, the oxide of lead serving to oxidise and carry off chemically or mechanically metals such as arsenic, antimony, or tin. The bullion, if in suitable proportions, is then ready for parting.
Outline of the sulphuric acid process as originally used in the New York assay office. The process comprised eight operations:
- Inquarting and granulating the bullion.
- Solution of the granulated bullion in sulphuric acid.
- Smelting of the fusion of the insoluble residue of gold.
- Condensation of the acid fumes.
- Reduction of the silver sulphates by copper.
- Sweetening and melting the precipitated copper.
- Treatment of the sulphate of copper.
- Treatment of bye products.
The alloys were made so as to contain from two to four of silver to one of gold. This was then melted. If iridosmine happened to be present, the parcel which contained it was melted with four or five times its weight of silver, mainly to decrease the density of the alloy; the platinum metals sank to the bottom. The molten alloy of silver and gold was then slowly poured out, taking care to leave the heavy metals in the bottom. More silver was added, and the process repeated until at last the amount of gold remaining was trifling. The “King,” or separated portion of the silver-gold alloy was then dealt with by itself, and the silver parted from it. The alloy of gold and silver was again melted and poured from a height of a few feet with a wavy motion into a vessel filled with water, which was kept cool.
The granulated bullion was introduced into cast iron pots of the following dimensions:—45in. diameter, 26in. deep, holding about 160 gallons of acid. Each pot is provided with a hemispherical hood, lined with lead, and connected on to the acid condensing chamber. Openings in the hood are provided for stirring the granulations or introducing material.
The charge of alloy was from 300 to 400lbs. and the amount of acid used was usually four times the weight of the alloy. After being boiled several hours, the solution was run off by means of a siphon, care being taken not to remove any gold. This was attained by turning up the end of the siphon for about three inches. Fresh acid is introduced, and the gold is withdrawn by means of a perforated ladle, and placed in a scoop. This gold was then placed in a smaller pot and more acid added and boiled. This operation was repeated five times. The gold was then placed in a tub and washed, first with cold water and then with hot. Very often lead sulphate is left with the gold, but by carefully running a stream of water over the gold, placed on an inclined slab, it may be washed away mechanically. After this it is boiled again with sulphuric acid if necessary, until, on melting, the bullion is brought up to 998 fine. In all about seven boilings with acids were required, the operations taking five days.
The Gutzkow Refining Process
The next improvement in the parting of gold and silver is due to Gutzkow. This was introduced into the San Francisco Assaying and Refining Works in 1867. Briefly, it consists of parting the alloy with strong sulphuric acid in iron pots, removing the solution containing sulphate of silver, diluting and cooling this to cause a deposition of crystals of the same; the acid thus freed from the sulphate of silver is used over again. The sulphate of silver itself was reduced by running a saturated solution of ferrous sulphate over it.
For this process when the alloy was rich in gold, such, for instance, as from two parts of gold to three parts of silver, it was granulated before parting; when it was poor in gold, such as when it contained from one of gold to five or more of silver, it was introduced into the acid in bricks or bars. Solution took place just as readily in the latter case, because there was less need for regulating the temperature in order to avoid frothing. Full descriptions of this process are to be found in Eggleston. Gold and Mercury, vol. II., p. 775, and in Percy, Silver and Gold, p. 479, and since the process has been improved upon nothing further need be said about it here.
The improvements made by Gutzkow on his process consist mainly in decomposing the crystals of sulphate of silver by heating them in a crucible with carbon. The improved process is in use in several parts of Australia.
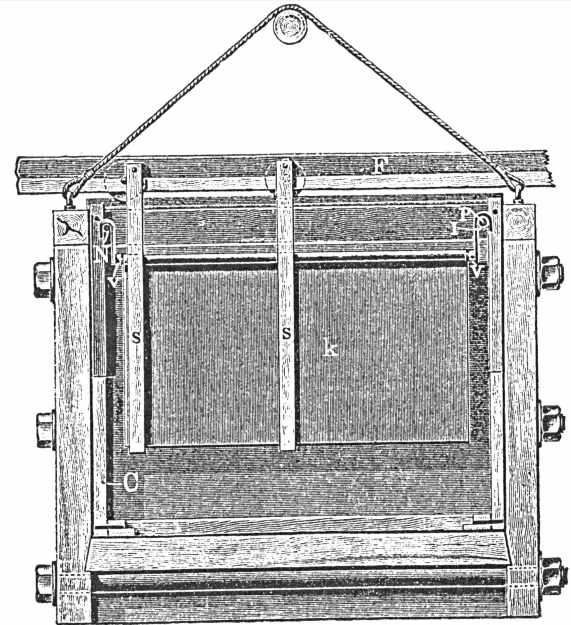
The following description is taken, mainly from a paper by Mr. G. H. Blakemore. The requirements are:
- One 400 gallon tank set on a stand eight feet high. This tank supplies the necessary water; either hot or cold.
- A cast-iron parting kettle, with movable cast-iron hood. The kettle should hold 1000lbs. of strong sulphuric acid. It is set over a fireplace.
- A small lead condenser to catch sulphuric acid and other vapors from the kettle. This is 10 feet x 7 feet x 5 feet, and is built with 4lb. to square foot lead.
- Two cast-iron pans. 6 feet x 3 feet x 1 foot.
- Two lead lined wooden boxes, with antimonial-lead plug cocks. Boxes are 2 feet x 18 inches x 18 inches.
- One lead lined wooden box, 6 feet x 1 foot 6 inches x 1 foot 6 inches, to hold precipitated copper.
- One recovery box, eight compartments, made of 2″ soft pine, each compartment 2 feet wide, 4 feet deep, 4 feet long. This is filled with scrap iron.
- Fire drying pan of cast-iron, 5 feet x 2 feet 6 inches x 6 inches deep, set over fireplace.
- Three leaden buckets to hold acids.
- One iron paddle, 4 feet long, of ¾ inch round iron.
- One iron ladle, to hold about one pint, ¾ inch iron handle.
- Two trowel-shaped tools.
- Six wooden paddles, one inch round handle, blade of paddle, 6 inches x 4 inches.
- Set of hydrometers.
- Melting furnace for gold.
- Rubber gum boots and gloves.
- Steam pipes with rubber connections and lead pipes.
- Water pipes to various places, also rubber hose and roses.
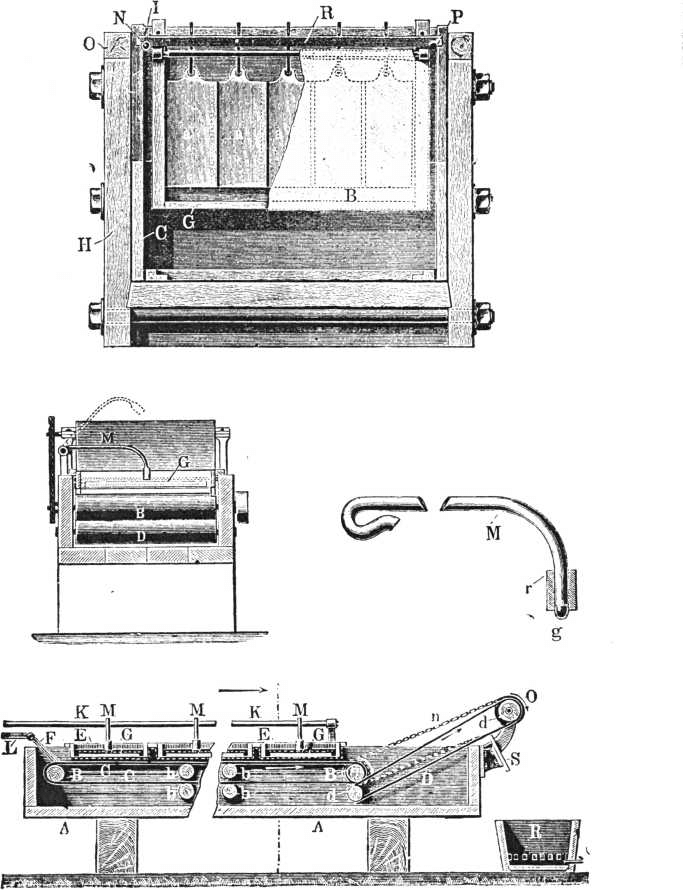
A charge for such a plant is 200 lbs. dore bullion or thereabouts. From 1¼ to 1½lbs. of concentrated acid per lb. of bullion is placed on the dore bars. The acid is boiled for about two hours. Two jars of the acid obtained at a subsequent stage are added and boiling continued for from three to four hours, when all the silver will be found in solution. The kettle is then filled up to within about an inch from the top with the acid before mentioned, so that about four pounds of acid are present for every pound of dore charged. When the cold acid is added there is a copious precipitate of bisulphate and sulphate of silver. In order to get this into solution it is sometimes necessary to re-heat the solution. When the silver has dissolved, the ladle is used, and the gold, which settles in a pocket cast in the bottom of the kettle, removed. When the gold powder is removed, the kettle is allowed to stand half an hour, and the contents are siphoned out by means of a piece of bent gas tube pipe, to within two inches of the bottom, into a cast-iron pan. Steam is then passed into the pan through a leaden pipe. The steam serves to dilute the acid, so that on cooling only the hard yellow crystal of sulphate of silver and not the white mushy crystals of bisulphate will form. The condition of the liquor is tested periodically by removing a drop with a glass rod on to a spotting plate. If bisulphate forms the liquid will solidify on cooling like a drop of candle grease. When the proper state of dilution is reached there will be a few yellow crystals surrounded by the liquid acid. The specific gravity is 1.66 or about 60 per cent, sulphuric acid. The time taken for steaming is 10 hours, when fresh acid is used for adding to the alloy, but three hours when the mother liquors from the crystals are used. The pan is then allowed to stand for 24 hours, or less if it is cooled by a water jacket. The mother liquor is then run off, and the crystals of silver sulphate are shovelled out into the leaden box. The crystals are washed with hot water, which dissolve the iron and copper sulphates, by decantation and stirring, then in a box on wheels, lead lined with a false bottom covered with strips of unbleached calico. Hot water is run on and about four washings given. The crystals are then removed to the drying pan; they become dry in about two hours. The dry sulphate of silver is mixed with about 4 per cent, to 5 per cent, of its weight of ground coke or charcoal and heated. In about four hours it is reduced to metallic silver.
Ag.2 SO4 + C =Ag.2 + SO2 + CO2.
It is advisable to put about 5lbs. weight of fireclay or other powdered siliceous material with the silver in order to prevent the retort from being eaten through at the line of the molten charge. A retort will hold about half the weight of sulphate of silver as compared with the molten metal. The silver obtained varies in fineness from 985 to 998.3. It is usually re-melted in a new cupel and brought up to 998. The washings from the sulphate of silver are run through a box containing precipitated copper, which precipitates the silver they contain. The copper entering into solution is precipitated in a box containing iron. The silver precipitate, mixed with a little copper, is put in a box, about 20 per cent, by weight of sulphate of silver crystals is added. This serves to remove the copper still present.
Ag.2 SO4 + Cu = Cu SO4 + 2Ag.
This is well washed with hot water, and the wash waters passed through the usual copper precipitating box. The sponge of silver is pressed, dried and smelted.
The gold after being washed is subjected to a second boiling with sulphuric acid, in the proportion of one of gold to eight of acid. It is boiled for about three hours. While still hot the gold is ladled out into a lead lined box, and washed with hot water until free from silver. After washing it is melted in crucibles, borax being added as a flux. The cost of a plant turning out 200lbs. of dore bullion every twenty-four hours is stated to be £550, and the cost of parting from 0.19d. to 0.21d. per ounce.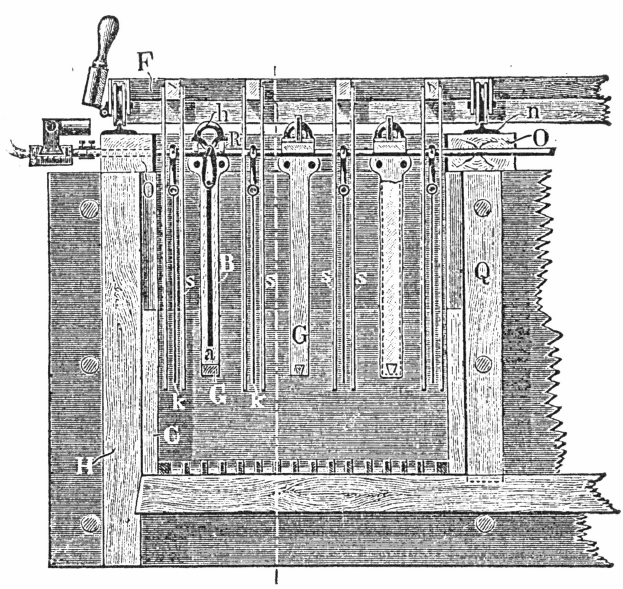
At the Broken Hill Proprietary works, at Port Pirie, practically the same method is adopted, the only difference being in a few details, such as in the latter there are three kettles in a row, the end ones being for the solution of the bars and the centre one for boiling the gold left a second time. Also the gold is not fished out with a ladle until the solution carrying the silver is siphoned off into the cool iron tanks. The tank is cooled by being surrounded by a water jacket, through which cold water runs. The silver sulphate crystals are washed with cold water, the washings are run over copper plates where the silver is precipitated, the copper in solution is precipitated on iron. The mother liquor, from which the silver sulphate crystals have separated, is concentrated by being run into a lead lined vat, where it is heated by means of a steam coil. The gold, after the second boiling in sulphuric acid (See “Australian Mining and Metallurgy,” Donald Clark) is washed with water. It is then boiled in hydrochloric acid, to remove the iron and lead salts, and smelted. It runs about 9912 fine. There are minor modifications of this process in use at various refining works. In some the gold left after the first treatment with acids is dissolved in aqua regia, and precipitated by ferrous sulphate, in others the gold is fused with a small quantity of bisulphate of sodium and sulphuric acid; strong acid is then added to dissolve out any sulphate of silver, and the resulting bullion is washed, fluxed with nitre, and smelted. The bisulphate has the effect of dissolving the platinum present. This is recovered by smelting the slags, consisting mainly of sulphate of soda, with litharge and charcoal, a platiniferous lead is produced which is cupelled. The alloy obtained is treated with aqua regia, and the platinum precipitated from the solution with ammonium chloride as chloroplatinate of ammonium. This is ignited and spongy platinum remains. By fusing the gold precipitate left on parting with nitre for some hours the platinum enters the slag as platinate of potash.