Table of Contents
The recovery of the precious metals like gold and silver from the cyanide solution is almost universally accomplished by precipitation with zinc, either in the form of fine threads or of dust (the condensed fume recovered in the process of retorting the metal).
The phenomena of precipitation are essentially electrical and may be traced to the action set up by two metals of different potentials forming a galvanic couple, with the cyanide solution as electrolyte. The chemical changes theoretically accompanying this action are expressed by Park in the equation:
2KAg(CN)2 + Zn = K2Zn(CN)4 + 2Ag
and Julian and Smart apparently assume the same reaction though they do not give the equation. According to this 0.166 parts by weight of zinc should be needed to replace 1 part of gold and 0.303 parts of zinc to replace 1 part of silver. Clennell, however, prefers the reaction
KAg(CN)2 + 2KCN + Zn + H20 = K2Zn(CN)4 + Ag + H + KOH,
in accordance with which it would require 0.332 parts of zinc to 1 part of gold and 0.606 parts of zinc to 1 of silver. In favor of the former equation it may be noted that the normal practice in the Pachuca district of Mexico when using zinc dust shows a consumption of zinc of not over 1 pound per pound of bullion and often as low as 0.8 lb. of zinc, while with the Crowe Vacuum Process even lower zinc consumptions have been obtained. Now by Clennell’s equation the theoretical proportions would be 0.6 of zinc to 1 of silver, and it would be rather surprising if the practical and theoretical figures were to approach so close to one another, especially when it is remembered that both the free cyanide and the free alkali in the solution tend to attack the zinc and dissolve it, and also that there is usually more or less unused zinc present in the precipitate after cleaning up. On the other hand Clennell’s reaction would better agree with the admitted fact of the difficulty of producing precipitation without the presence of a fair amount of free cyanide and would also explain the observed rise in alkalinity after precipitation.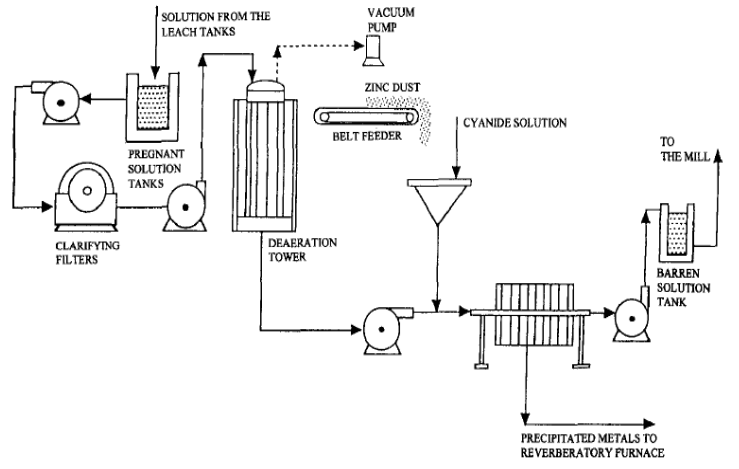
Park’s equation does not show the liberation of hydrogen as being an essential to the reaction, though the dissolution of some of the zinc by free cyanide and free alkali may be sufficient to account for the almost universal generation of that gas in the well-known copious amounts.
4KCN + Zn + 2H2O = K2Zn(CN)4 + 2KOH + H2
2KOH + Zn = K2ZnO2 + H2
Whatever be the exact terms of the reaction zinc replaces the precious metal in the solution and there is a tendency for this zinc to accumulate in the stock solutions after repeated use. This accumulation, however, in practice only proceeds to a limited extent and this fact has been the subject of speculation. Of course, a small quantity of the stock solution with its burden of zinc is continually passing out of the plant in the residue moisture but where the usual water washes are used this will not account for the whole effect noted. Some zinc is no doubt precipitated as sulphide by soluble sulphides formed from the ore during the dissolving treatment, but it would seem that both these causes need to be supplemented by something further. The writer is inclined to account for it by the effect of the lime added to the pulp upon the double zinc cyanide. It is almost universally admitted that there is a considerable rise in the free cyanide strength of the precipitated stock solution when it comes in contact with lime and this may be accompanied by a precipitation of the zinc in an insoluble form, either as calcium zincate,
K2Zn(CN)4 + 2Ca(OH)2 = 2KCN + Ca(CN)2 + CaZnO2 + 2H2O;
or, as Walter Virgo suggests, in presence of carbonic acid, a double carbonate of zinc and calcium may be formed
K2Zn(CN)4 + 2Ca(OH)2 + 2CO2 = ZnCa(CO3)2 + 2KCN + Ca(CN)2 + 2H2O
White Precipitate
It often happens, more especially in the precipitation of solution containing less than 0.1% of free cyanide, that a white deposit is formed on the zinc shavings, generally in the shape of little excrescences adhering to the metal and in its extreme manifestations cementing the zinc together in solid white masses quite impervious to the solution. It is usually found to consist principally of zinc hydroxide with varying amounts of zinc cyanide and zinc ferrocyanide and sometimes calcium compounds. Its formation is sometimes said to be promoted by a high lime content in the working solution but the writer has never seen any evidence to support this view. This white precipitate is a nuisance because it both interferes with proper working of the zinc boxes and also yields a low-grade product which is difficult and expensive to melt.
As to the remedy it is not easy to make suggestions applicable in general apart from a consideration of the individual conditions. A higher strength of free cyanide and free alkali will usually be effective in preventing its formation, but circumstances may not always warrent such an increase. In that event the next best thing is, probably, to dress the boxes more frequently so as to detach the coating from the zinc and keep a clean metallic surface exposed, preventing also the formation of patches and layers impervious to the flow of solution.
What is the role of Oxygen in Gold Precipitation
In regard to this question some interesting facts have been noted in connection with the recently devised method for determination of oxygen in working solutions already described in the chapter on ” Testing and Analysis of Solutions” page 48. In the course of the article by H. A. White referred to and the discussion thereon it is shown that (1) the oxygen contained in the solution entering the zinc boxes is rapidly eliminated by the reducing action of the hydrogen formed and that the rate of precipitation increases as the oxygen content is diminished, and (2) that the formation of white precipitate seems to be a function of the amount of oxygen present, and that when the solution reaches a point where the oxygen has been practically eliminated, no white precipitate is formed even under conditions normally favorable to its production. The first observation tends a priori to demonstrate the advantage likely to be derived from a mechanical removal of the dissolved oxygen from the solution before precipitation while the latter suggests the possibility that by such a preliminary elimination of the oxygen the formation of white precipitate might be entirely prevented.
The Crowe Vacuum Process
It is interesting to note that almost at the same time that the action of oxygen in precipitation was attracting attention on the Rand, Thomas B. Crowe at the Portland Mine, Cripple Creek, was perfecting a process for the mechanical removal of dissolved oxygen from solutions, arguing that precipitation being a reducing process the less oxygen present the more favorable would be the conditions for the reaction. The invention consists in spraying or otherwise disintegrating the solution in a vacuum chamber by which means the dissolved oxygen is removed before the zinc dust is added, resulting in a reduction of the zinc consumption and also in some instances a reduction in the free cyanide loss which accompanies zinc precipitation. The process which is at its best when used in combination with zinc dust has shown savings in zinc amounting to two-thirds of the former consumption. In the case of zinc shaving precipitation its benefit is not so marked on account of the extreme rapidity with which the solution re-absorbs oxygen, but even here in the few instances in which it has been tried it appears to have resulted in a saving of zinc coupled with the possibility of obtaining good precipitation from solutions too low in free cyanide to precipitate under normal conditions.
How Lead increases Gold Precipitation by Zinc
The principle of coating the zinc with a film of lead to increase the activity of precipitation was patented by MacArthur in the early days of the process but it was not till the occasion arose for precipitating the exceedingly dilute solutions obtained from slime treatment on the Rand that the process attained to commercial prominence. When the decantation process of slime treatment was first mooted the impossibility of precipitating the solutions by zinc was assumed and the Siemens and Halske electrolytic process was considered indispensable. This had its drawbacks, however, and in the search for a substitute it occurred to W. K. Betty to try the zinc lead couple. He found that by its use, combined with a drip of strong cyanide solution into the head of the box he could precipitate satisfactorily solutions carrying as low as 0.005% in cyanide. The ordinary zinc shavings were dipped in a solution of lead acetate containing from one to five per cent, of lead, thus coating them with a deposit of spongy metallic lead. This zinc was then placed in the extractor box and covered with solution as soon as possible. A barrel containing concentrated cyanide solution was placed at the head of the box, and as soon as the pregnant stock solution was turned in a small drip of this strong solution was allowed to run in with it, and maintained for the first twelve hours. Julian and Smart state that this feature was later on abandoned.
When working with this process even under the most favorable conditions it was not found possible to maintain effective precipitation for more than five or six days after starting, and when the action became sluggish the zinc was transferred to the sand plant precipitation system and replaced by new.
How Copper affects gold precipitation
Copper sometimes give trouble by forming a continuous and adherent skin over the zinc and stopping further precipitation of the gold and silver. This effect is more often observed when the solution is low in free cyanide, and in strong solutions copper interferes very little with the proper working of the boxes. When trouble occurs from this cause the zinc lead couple may be found a valuable aid in counteracting it. Park states that its effect is to keep the copper in solution allowing only gold and silver to be precipitated but at the Creston Colorada mine in Sonora, Mexico, a system was in vogue, invented by Mr. Higgins, the metallurgist, of precipitating the gold and silver with a minimum of copper, out of the strong and rich solution on ordinary zinc shavings and passing the weak and low grade solution through boxes in which the zinc was heavily coated with sponge lead, for the purpose of throwing out the copper and preventing its accumulation in the stock solution, which it was said to accomplish very satisfactorily.
Should the presence of copper be found an insuperable obstacle to precipitation by zinc shavings, the zinc dust method will usually not be open to the same objections. The electrolytic process also may sometimes be effectively employed, either for recovering the gold, silver and copper together in the form of base bullion or for cleaning the stock solution of its copper content after recovering the gold and silver by the zinc dust method.
There is an interesting process in use at Dayton, Nevada, for precipitating the solution resulting from the treatment of the accumulated coppery tailings from the old pan amalgamation mills of Virginia City. The solution after the necessary contact with the ore is heated to about 200 deg. F. and strengthened with fresh cyanide before the zinc dust is added. By this means not only is no trouble experienced about precipitating the silver, but a large proportion of the cyanide combined with copper is said to be regenerated.
Method of Working When Using Zinc Shavings
The extractor box is a long narrow box made of wood or steel and divided up into from five to ten compartments by partitions, and baffles so arranged that the solution rises through the column of zinc in each compartment and descends through the narrow space between the partition and baffle.
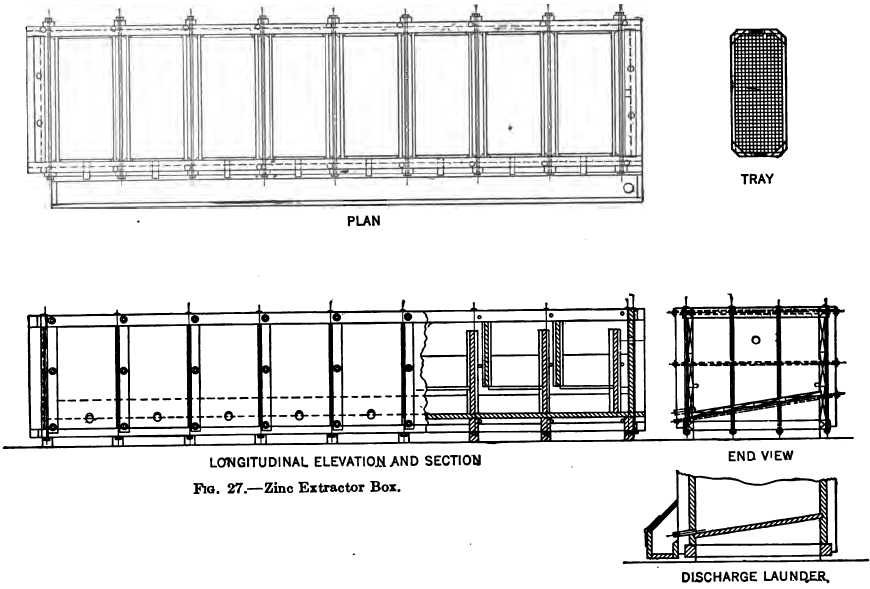
A tray whose bottom is composed of iron screening of about ¼ inch mesh rests on cleats six inches above the bottom of the box so as to allow space beneath for the collection of the gold and silver sludge as it becomes detached from the zinc.
Should the boxes be made of steel it is better to keep the interior surface well covered with P. and B. paint as it has been found that when the solution contains much caustic soda, a couple is formed between the iron and the zinc, resulting in a marked increase in zinc consumption. The same applies in a less degree to the use of iron screening on the zinc trays and some metallurgists have substituted screens made of fibre or some non-metallic material.
The zinc filaments or shavings are packed in on top of the tray almost to the height of the compartment, care being taken that the whole space is uniformly filled especially in the corners. A good method, devised by L. M. B. Bullock, is to wind the shavings off the lathe into wisps or hanks about three inches in diameter and an inch or two longer than the measurement of the compartment. When charging a box these hanks are doubled over at each end to the exact size and laid carefully side by side and close together forming a layer of one hank in thickness with all the threads parallel to one another. Another layer is then placed on this but transversely, and so on till the compartment is filled. The idea of making the hanks a little too long and then doubling the ends is to give increased compactness and density at the sides and corners where the greatest channelling occurs.
A fine thread-like shaving is usually preferred to the ribbon form and the thickness should be such as to give the maximum surface per unit of weight without making it so slender as to disintegrate rapidly into short pieces under the action of the solution. A width of from 1/500 to 1/800 of an inch is probably the most useful.
The zinc, especially in the first three compartments should be dressed frequently. If the solution is strong in cyanide and rich in metal this may need to be done every day and the lower compartments every four or five days.
In dressing and also in cleaning up the most approved practice is to move up the zinc from the lower compartments to replenish that which has been dissolved in the upper ones, filling the spaces thus left vacant with new zinc. By doing this the bulk of the precious metal is concentrated and maintained in the head compartments, minimizing the risk of precipitate being carried over the end of the box mechanically, and keeping a body of new clean zinc for stripping the last traces of metal from the solution. Moreover, it is only by this means that the partly consumed zinc can be completely used up. Even so there is always a certain amount that breaks into short pieces which tend to mat together and render the compartment impermeable to the solution.
When the amount of the broken zinc is not excessive it can be disposed of by sprinkling a thin layer between each layer of long zinc in the upper compartments, but if, as sometimes happens, the quantity grows to unmanageable proportions, screen trays may be placed on top of the head compartments just below the level of the solution and the short zinc laid in a thin layer on the screens, being removed each day and washed to detach the adhering precipitate and expose fresh surfaces to the action of the solution. Another useful device for working up these zinc shorts is a small agitator tank about three feet diameter and two feet deep with a circular overflow launder. Between this launder and the rim of the tank is placed a circular screen of 30- to 60- mesh wire cloth. The short zinc arising from each day’s cleanup of the boxes is placed in the tank and a small stream of pregnant solution run in, the stirring gear keeping the whole charge in continuous agitation. As the rich solution dissolves the zinc and precipitates the gold and silver, the stream of solution continuously flows out through the circular screen into the annular launder, carrying with it the precipitate. This stream enters the head of one of the precipitation boxes and deposits its suspended material there, its soluble content being also completely precipitated as it flows on down through the zinc shavings.
In dressing the boxes and especially in cleaning up it is most important not to allow the zinc to remain exposed to the air, as it gets hot and oxidizes rapidly, resulting in a considerable increase in zinc consumption and also favoring a formation of the obnoxious “white precipitate.”
Capacity of Zinc Boxes
The total cubic capacity of zinc boxes necessary varies with the quantity of solution and its metal content and also with the method used for packing them with zinc shavings. The following were the conditions at Butters Copala Mines where the boxes were packed by the Bullock method.
There were two boxes of eight compartments each; both were 24 inches deep but one was two feet wide and the other, one foot six inches; the width of the compartments was in every case fifteen inches.
Average value of solution before precipitation $1.01 in gold and 3.41 oz. in silver. Average value of solution after precipitation, at the rate of 15 tons per hour traces, at the rate of 25 tons per hour, $0.06 gold and 0.03 oz. silver.
Daily tonnage of solution…………………………………………………………………………………..360 to 600
Gross capacity of the two boxes, cu. ft…………………………………………………………………70
Cu. ft. in boxes for each ton of solution treated daily…………………………………………….0.19 to 0.12
Space actually occupied by zinc shavings, cu. ft…………………………………………………..49.5
Solution per day for each cu. ft. of zinc, tons………………………………………………………..7.2 to 12.1
When the boxes are packed by the usual method of throwing the loose shavings in, armful by armful, and pressing them down, the space necessary may be three, or four times that allowed at Copala and for low-grade gold solution especially those from slime treatment a cubic capacity ten times as great as that quoted may be necessary.
Zinc Dust Precipitation
The method of applying the zinc to the solution in the form of dust or fume was first introduced by Sulman and Pickard but it did not attain to a wide commercial importance until C. W. Merrill devised his modification of it for the Homestake Mine. The special point about the latter process consisted in adding the dust to a flowing stream of solution instead of, as heretofore, to an agitating cone or tank. The dust is released from its receptacle by a feeder whose speed is adjustable and falls into a mixer or emulsifier where it is thoroughly wetted and then passes, usually, directly into the suction pipe of a triplex plunger pump, discharging into the precipitate press. The action is so rapid that it is practically complete by the time the emulsion reaches the chambers of the press but as a measure of precaution the delivery pipe is lengthened as much as possible, sometimes extending to 200 to 300 feet before reaching the press.
If desired, the special triangular press designed for the purpose by the Merrill Metallurgical Company may be used but the writer has found that the ordinary square type of press answers the purpose equally well, the only thing necessary being to ensure that the filtering area is large enough to allow the necessary rate of flow per hour to pass through it, such area being for practical purposes about 1 sq. ft. per ton per day.
In order to obtain a complete precipitation, that is, to produce an effluent assaying only 2 or 3 cents, a rather heavy feed of zinc dust is sometimes necessary resulting in a low-grade precipitate for melting. This is more especially noticeable in the treatment of low-grade gold ores and is less in evidence where the solution contains several ounces of silver per ton. At the Homestake it is often found advisable to run a drip of lead solution, either nitrate or acetate, at the point where the zinc dust enters the stream, in order to intensify the action. At this mill the precipitate is so low in grade that it is first acid-treated and then dried and fluxed with litharge preparatory to being finally cupelled. By this means a bullion 980 fine is produced.
In order to reduce the quantity of zinc dust used while at the same time producing a barren solution for residue filter washes J. S. Colbath introduced a combination system at the mill of the El Rayo Mining Company. This consisted in carefully adjusting the supply of zinc dust and maintaining it at the lowest possible point so as to precipitate the greater part of the precious metals and allowing the effluent solution to assay as high as 20 to 40 cents per ton. A part of this effluent solution, just sufficient in quantity to provide barren washes for the ore residues was diverted and passed through one or more boxes of zinc shavings to strip the remaining assay values and produce a strictly barren solution. By this means an unusually low consumption of zinc dust was produced and the resulting precipitate was considerably higher in value than it would otherwise have been. The cost of maintaining the box of zinc shavings was small as only part of the daily solution tonnage went through it and it needed very little attention on account of the small quantity of precious metal deposited therein. In recent years, however, the zinc dust process has been so developed and improved that it is now hardly worth while going to the additional trouble of the double system.
Advantages of method of precipitating gold with zinc dust
- Zinc dust costs less per pound than zinc shavings.
- Less zinc is usually consumed per unit of fine bullion than in the case of zinc shavings.
- It is superior to shavings for precipitating coppery solutions because it does not afford the same opportunity for the copper to form an impervious skin over the zinc surface and stop further action by the solution.
- The laborious and disagreeable task of dressing and cleaning up the zinc boxes is avoided, and (5) greater security from pilfering the precipitate is effected.
With clean solutions high in silver it is possible, even when the El Rayo system is not used, to produce bullion which by direct melting assays over 900 fine with a consumption of zinc dust not over one part to 1 of fine bullion, by weight; indeed in some of the Pachuca plants the zinc dust consumption has been less than 0.8 kilo per kilo of fine bullion.
