Table of Contents
 When cyaniding a precious metal ore containing pyrrhotite, various compounds such as, thiosulphate, thiocyanate, ferrocyanide and soluble sulphide are formed and found in the cyanide solution. Soluble sulphides, if present at all, are found only in relatively small amounts; on the other hand thiosulphate, thiocyanate and ferrocyanide have been found in solutions in amounts of >0.25%. At times they have been suspected of interfering with the determination of free cyanide by titration with silver nitrate.
When cyaniding a precious metal ore containing pyrrhotite, various compounds such as, thiosulphate, thiocyanate, ferrocyanide and soluble sulphide are formed and found in the cyanide solution. Soluble sulphides, if present at all, are found only in relatively small amounts; on the other hand thiosulphate, thiocyanate and ferrocyanide have been found in solutions in amounts of >0.25%. At times they have been suspected of interfering with the determination of free cyanide by titration with silver nitrate.
In cyanidation there is no hard and fast rule for the procedure followed in determining free cyanide; some operators, when titrating with silver nitrate, use no indicator other than the first appearance of opalescence i.e. the precipitation of AgCN; others use potassium iodide in various amounts, the precipitation of AgI indicating the end-point.
Series of titrations were run in which the effects of various compounds on the free cyanide determination were investigated both with and without potassium iodide present.
Effect of Sodium Thiosulphate
 A cyanide solution containing 0.026% NaCN and 0.001% CaO equivalent was prepared. 50 ml portions were pipetted into beakers containing weighed amounts of potassium iodide varying from 0.01 to 5.0 grip.; in one test no potassium iodide was used. To each was added 0.125 grm. Na2S2O3; thus each solution contained 0.25% Na2S2O3. The solutions were then titrated with silver nitrate. A second series of similar tests was run in which the effect of 0.10% Na2S2O3 was investigated.
A cyanide solution containing 0.026% NaCN and 0.001% CaO equivalent was prepared. 50 ml portions were pipetted into beakers containing weighed amounts of potassium iodide varying from 0.01 to 5.0 grip.; in one test no potassium iodide was used. To each was added 0.125 grm. Na2S2O3; thus each solution contained 0.25% Na2S2O3. The solutions were then titrated with silver nitrate. A second series of similar tests was run in which the effect of 0.10% Na2S2O3 was investigated.
- Catalytic leaching of silver with ferricyanide-cyanide solution
- Solvent extraction of copper & cyanide from waste cyanide solution
- Cyanide titration reference guide
The chemistry of cyanide solutions resulting from the treatment of ores by Clennell, J. E. (John Edward)
Results show that when thiosulphate is present in a cyanide solution, titration with silver nitrate indicates a very high figure for the free cyanide content if no potassium iodide has been added; depending upon the amount of thiosulphate, the figure may indicate several times as much cyanide as is actually present. Small additions of potassium iodide reduce the error considerably, but it is not until 0.5 to 1.0 grm. of potassium iodide has been added that a reliable figure for the free cyanide content of a solution containing thiosulphate is obtained. When a large excess of potassium iodide is added, the figure for free cyanide again is inaccurate, being higher than the amount actually present.
The reasons for these discrepancies are as follows: With no potassium iodide present, the end-point of the titration is indicated by the first appearance of a precipitate of AgCN. This precipitate dissolves in thiosulphate according to the reaction:
2 AgCN + 3 Na2S2O3 = Ag2S2O3*2 Na2S2O3 + 2 NaCN,
the overall equation for the titration being:
2 NaCN + 3 AgNO3 + 3 Na2S2O3 = Ag2S2O3 * 2 Na2S2O3 + NaAg(CN)2 + 3 NaNO3.
This reaction proceeds to completion, i.e. until both cyanide and thiosulphate have reacted with silver ions. Until this reaction is complete there will be no precipitation of AgCN, and therefore no end-point.
When potassium iodide is added to the cyanide solution, the end-point of the titration is indicated by the first appearance of AgI. This AgI is only slightly soluble in thiosulphate, the precipitation of this compound being influenced by the amount of KI in solution. An excess of iodide ions will favor the precipitation of AgI and thus drive the reaction to the left:
2 AgI + 3 Na2S2O3 <–> Ag2S2O3*2 Na2S2O3 + 2 NaI
Under these conditions a reasonably accurate determination of free cyanide in the presence of thiosulphate can be made. However, when a large amount of potassium iodide is used an entirely different reaction takes place. Silver iodide dissolves in concentrated solutions of KI forming the complex K3AgI4:
AgI + 3 KI = K3AgI4
The end-point is delayed until the KI will dissolve no more AgI. According to researchers, a 0.34 molar solution of potassium iodide dissolves only 0.00036 mols of silver iodide whereas a 1.94 molar solution dissolves 0.198 mols of silver iodide.
Effect of Sodium Sulphide
When a cyanide solution contains soluble sulphide, a brownish black precipitate of silver sulphide is formed when silver nitrate is added. This precipitate, even in minute amounts, masks the end-point in the cyanide determination. The usual procedure when determining cyanide in solutions containing soluble sulphides is to add a pinch of litharge (about 0.5 grm.) and stir for about a minute. This precipitates the sulphide on the litharge as lead sulphide which is filtered off, the filtrate being then titrated for cyanide and alkalinity in the usual manner. In order to determine whether or not this preliminary treatment with litharge affects the subsequent determination of cyanide and alkalinity, the following experiment was run: A solution was prepared containing 0.026% NaCN, 0.06% CaO, and 0.004% Na2S. The sulphide was removed by treatment with 1 grm. of litharge and the filtrate titrated first for cyanide using 0.5 grm. of potassium iodide as indicator, and then for alkalinity by titration with oxalic acid using phenol phthalein as indicator. The results checked the original composition of the solution thus indicating that preliminary treatment with litharge had no effect on cyanide or lime in solution.
Effect of Thiocyanate
A solution was prepared containing 0.026% NaCN and 0.25% NaCNS. Portions were titrated with silver nitrate varying the amount of potassium iodide added, from zero to 1.0 grm. In every case the result showed 0.025 to 0.026% NaCN, thus showing that thiocyanate has no effect on the determination of cyanide by silver nitrate titration.
Effect of Ferrocyanide
A solution was prepared containing 0.026% NaCN and 0.25% Na4Fe(CN)6;. Portions were titrated varying the amount of potassium iodide as in the previous tests. In every case where potassium iodide was used, 0.026% NaCN was indicated by the titration. With no potassium iodide present a high result, 0.033% NaCN, was indicated thus demonstrating that for an accurate cyanide determination in the presence of ferrocyanide, potassium iodide should be used as an indicator.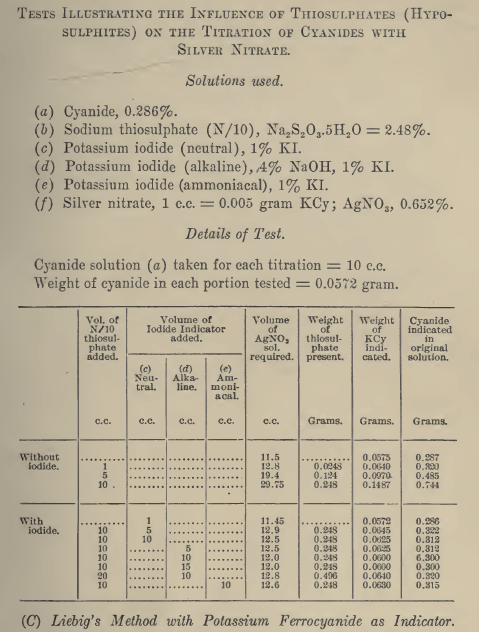
https://www.911metallurgist.com/estimation-of-free-cyanide-by-means-of-a-solution-of-iodine-in-iodide-of-potassium

