Table of Contents
The treatment of auriferous ores presents several challenges to the metallurgists and plant operators due to there are some factors that have an important impact on the process. Without any doubt the mineralogy is the key aspect of the process performance. Basically, there are several options to be considered at the moment of evaluate the ore. The first alternative is a gravimetry process and to reprocess the tails by flotation and/or cyanidation. No matter the number of processes involved because the mineralogy is different in each deposit and governs the success of the precious metals recovery. The resolution of problems in process performance requires an understanding of the ore mineralogy. The next lines explain the main impact on the process.
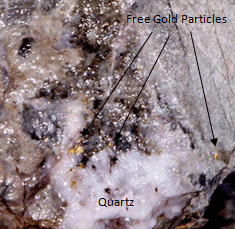

Native gold hosted by quartz Gold grain detected at 63x
Gold ores:
Basically when a gravity process is able to recover more than 50% of gold, the potential presence of free or native gold is important. If the gold recovery is low, it is necessary to consider more alternatives such as flotation and cyanidation. There is a usual or common classification about gold ores based on its recovery by cyanidation. If the recovery is more than 90%, the ore is considered as free-milling ore. In the same way, a refractory ore is considered if the material gives low recovery by cyanidation and some extra process will be required to enhance the process performance.
In order to improve the performance is important to carry out mineralogical studies of gold behavior in various ores and metallurgical products in order to classify the ore and address issues and problems related to gold ore processing. It is widely used as a predictive and trouble-shooting tool in gold ore processing, and provides useful information on process selection, flowsheet development, recovery improvement, and reagent consumption optimization.
The main gold ore types are listed below:
- Placers
- Leachable
- Weathered
- Silver-gold
- Iron sulphides minerals
- Base metal sulphides
- Gold tellurides
- Non-sulphide gangue
- Carbonaceous material
- Arsenic minerals
- Antimony minerals
Gold Particle Size:
In order to establish a gold particle size classification, gold particles can be classified as visible, microscopic, submicroscopic and surface bound. The first class is referred to free gold particles detected on placer deposits or some hydrothermal deposits. The human eye can identify these gold particles without difficulty. The liberation of gold particles is close to 100% and the presence of any carrier is not necessary is most the cases. These kind of gold particles are affected by erosion, attrition and deformation. The latter one is probably a very important factor to be considered on gold recovery due to gold is malleable and particles can occur as flattened, square or any irregular form. These shapes are responsible for the metallurgical performance of gravity processes.
The second class includes the gold particles detected by using a regular microscope. It means that is not necessary a special microscope type. In this way we can detect free gold particles, Calaverite, sylvanite, krennerite, petzite, nagyagite and others. It believed that some gold deposits have a problem related to the decomposition of tellurides to oxidized compounds and native gold. This problem is major when the deposit content pyrite and/or arsenopyrite. The microscope helps to detect the presence of sulphides and gold particles associated or not. In this way we can study several ore types and the shape of gold particles. Even, some inclusions can be detected without having an important impact the host rock. The key factor is to detect the mineral with gold.
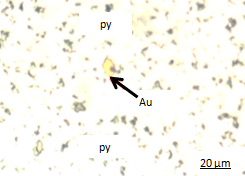
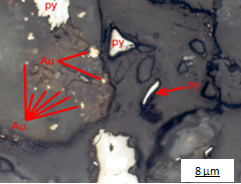
Figures show two different gold grain sizes with different degree of liberation
The third class is related to gold particles detected by special techniques. Perhaps one of the last techniques is the QemScan. Basically, the detection is possible by using microscopic scanning procedures. Gold particles are so insignificant is size that the observation under the microscope must be done several time in order to sure about the results. These gold particles are called invisible due to they are difficult to detect. Some carbonaceous deposits (Carlin) present these particles, but most the time base metal deposits present these gold particles. Perhaps, copper and copper-lead-zinc deposits are typical examples. The problem is noted when the head assay reports gold values and at the moment of detected the gold under a microscope, results are negative, even trying to pre-concentrate the sample. Gold particles are very small, 0.2 µm or smaller.
Probably, there are two sulphides mineral with the main probability of carrying gold particles, pyrite and arsenopyrite. These ores are treated most the time by flotation and/or cyanidation. If we consider flotation, gold values are reported on tailings (80-90%) and the relationship with pyrite and/or arsenopyrite is notable. The particles sent to the concentration or leaching process have an important effect. For example, coarse particles need fine liberation in order to make successful the process performance; porous particles will be useful to cyanidation because sodium cyanide solution can penetrate the particle and dissolves the gold; disseminated particles require fine liberation for flotation and cyanidation. Obviously, the regrind process has restrictions and some ores can’t be reground excessively fine as they need. In this case, the ore is considered refractory is the recovery is very low.
Surface bound particles are present in form of very small stains onto the minerals surfaces. They were formed during the mineralization process. In this way, the geochemical reactions have an impact on the formation of this type of gold particles. The main minerals that act as carrier of gold are iron oxides, clayed minerals and organic compounds.
Gold Metallurgy Performance
Although gold deposits mined around the world are different, there are some factors to be considered at the moment of performing metallurgical tests or study the problems during the operation.
- Particle size
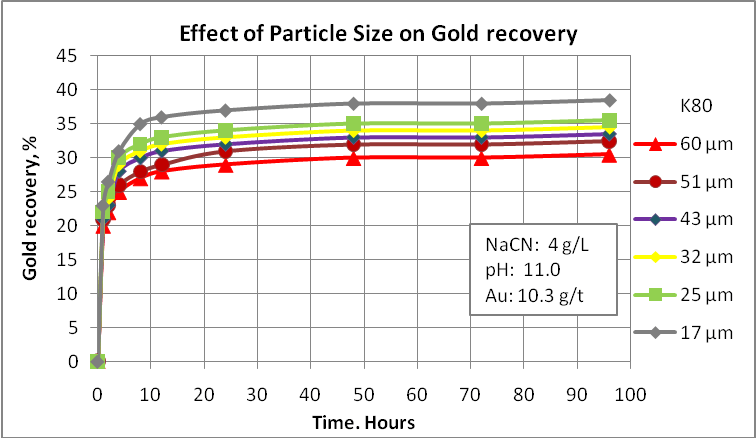
Probably this is the main factor to consider in any metallurgical evaluation. For example, native gold can be recovered by gravity equipment and the gravity tails needs to be reground to a finer size so that the net process can achieve a good performance. If gravity tails are processed by froth flotation, the flotation feed size must be ranging 70-75 passing 75 µm. If we considered cyanidation, the fineness of regrind is 85-90% passing 75 µm. Obviously, gold recovery processes have restrictions related to the gold particle size and no one can say that very fine particles are appropriate to achieve the best metallurgical performance. In this way, tiny particles such as 5 µm can create some problems during the cyanidation process. For this reason is important to evaluate the effect of the final grinding product on gold recovery in order to select the most appropriate size to use in the design. Also, the operative cost is directly influenced by the power consumption. Then, try to optimize this process is very important.
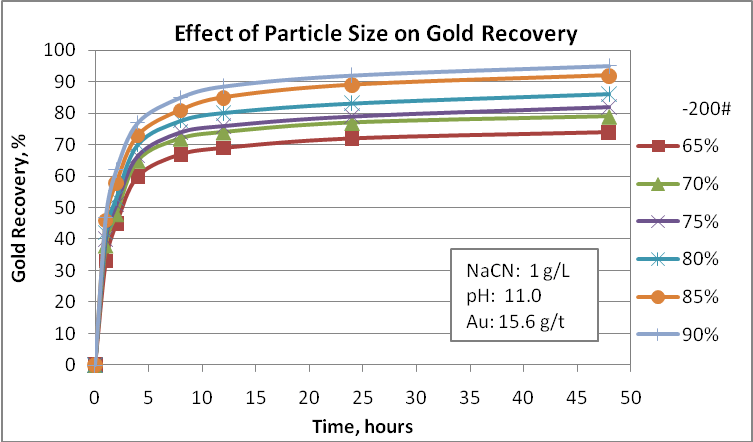
Cyanidation tests at different particle sizes
- Refractority
During the geological formation gold particles can be encapsulated inside minerals particles such as pyrite, arsenopyrite and some silicate minerals. Try to break the crystal structure and liberate the gold grains is sometimes impossible due to gold size is very tiny (0.1 µm) and comminution equipment is not able to liberate gold. Typically, this problem produces gold losses and the solution can be complex. Perhaps, cyanidation is the main process affected by gold particles occluded and encapsulated inside other minerals.
Encapsulated gold ore
- Submicroscopic particles
This item is a special case of the classic refractory problem, but with a very important difference, the problem is detected by microscopic devices. Previously was mentioned the effect of particle size on gold recovery. When there are tools to detect the problem at the beginning of the test work program, the metallurgist will be able to prepare a testing program considering this problem. The problem is basically gold liberation. As gravity concentration is not a good option, the next two alternatives are flotation and or cyanidation. The first one could concentrate part of the gold, but some losses will be related to two factors, gold particles liberation was not achieved and excessive presence of fine particles are a poison for the process due to they take part of the flotation reagents and modify the rheological properties of the slurry.
The success of cyanidation is related to the gold particles liberation obtained during the primary grind. If the problem is not very serious, the process could solve the problem, but if there are encapsulated gold particles, the process could not give an acceptable recovery.
- Tarnished and smeared particles
These two factors affect negatively gold recovery by flotation and leaching. Tarnished particles were obtained during the geological formation of the deposit by oxidation, dissolution, reduction, and precipitation processes. For example, some stains of iron oxides could be detected on sulphides particles. Then, there are two effects, first part of the particle is readily floatable and other not. This is a competition between the hydrophobic and hydrophilic areas on the same particle.
Smeared particles are obtained during the ore processing. The main source of these stains is the grinding process. Ball mill operates under different conditions, but there is a common problem, the production of iron compounds. Basically, sulphide minerals and free gold particles react with reagents in flotation and cyanidation. The efficiency of the process is influenced by particle surface changes resulting from the reaction of the grinding media and dissolved gases. In this way, gold recovery is governed by oxidation-reduction reactions in the slurry, although there is some doubt as to whether it is the oxidizing conditions or the oxidant which affect both processes. The level of oxidation of the particles is significant when flotation is performed with xanthates. This condition affects flotation.
It is well known that sulphide minerals are not stable in the presence of water and air and the condition of their surfaces, after primary grind, not only depends if the equilibrium was achieved, else on the oxidation rate. Typically, metallurgists and operators have not considered the effect of the grinding environment on the recovery process.
Nowadays it is possible to know the different types of compound deposited on mineral surfaces and how they were formed. Also, when there are detailed studies, the metallurgist can establish the relationship between pH and pulp potential in order to know the conditions that favor this problem.
- Silver-gold ores
When the silver/gold ratio takes values as high as 6-10, the ore will be processed by gravity concentration and flotation in order to get a silver-gold concentrate. There is a potential problem when the silver minerals are partially oxidized during the geological process. If the silver size particles of 3-4 microns, the problem is serious due to the easy oxidation of these particles. The problem can be detected by performing a mineralogical study. Basically, electrum and silver sulphosalts suffer this problem. They form silver sulphates and silver oxides. If part of these oxidized particles is recovered by entrainment, and the concentrate will be reprocessed by cyanidation, the final recovery will be affected.
It must be emphasized that the objective of processing a silver-gold ore is to obtain a concentrate with high silver contents. Normally, gold is not presence in high proportion, but its presence into the concentrate is important because gives an extra value to the concentrate.
- Cyanicides
It is important to detect the presence of some cyanide consumers. These minerals take cyanide and there is only residual part for leaching gold minerals. When they are present in high levels, the cyanidation process will not have high recoveries. Obviously, there is a critical point that must be detected by studying metal content and mineralogical composition. These undesirable minerals form cyanides and their formation generates operational and environmental problems such as tailings disposal or water balance. This problem increases operative cost and reduces gold recovery due is required higher cyanide addition to compensate the lack of cyanide to leach gold.
Without any doubt, the most serious problem is the presence of oxidized copper minerals and some secondary sulphides. Basically, there are several types of copper cyanide compounds which are formed under different chemical conditions during contact with cyanide. Some of them are
[Cu(CN)2]–, [Cu(CN)3]2- and [Cu(CN)4]3-. The Copper cyanide complexes have limited ability to dissolve gold, thus the free cyanide concentration in solution must be maintained at a level which ensures that the maximum amount of gold is dissolved. It a common practice to analyze the pregnant solution by different compounds and especially cyanide and copper in order to determine the presence of copper compounds.
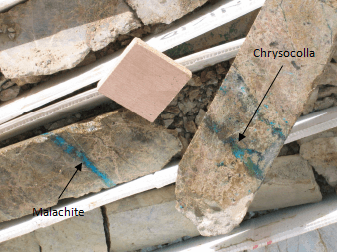
Malachite and chrysocolla are oxidized copper minerals
Other problem is the presence of free sulfur or sulphides. Cyanide reacts with sulfur to produce thiocyanate and this situation favors the oxidation of reduced sulfur compounds. And consequently, the operation needs to increase the pH in order to don’t release hydrogen cyanide.
- Gangue
The term gangue is referred to non-valuable minerals present in the ore. These minerals are variable and the potential is related to the specific mineral type. Carbonates are characterized by reacting with acid solutions and its relative softness. If we need to include an acid stage in the flowsheet, the acid consumption (e.g. sulfuric acid, nitric acid) will be extremely high and the chemical compounds produced by these reaction will be deposited on several parts creating some disturbs. If the ore needs to be treated by flotation, the excessive quantity of fine material is a problem because flotation reagents are taken by these tiny particles. Then generation of slimes must be noted during the test work program and the mineralogy study must indicate the type of carbonates present in the ore.
Carbon or any type of carbonaceous matter creates problems during flotation and cyanidation, especially for the second. Carbon absorbs flotation reagents (xanthates, frothers) and the dosage must be increased in order to have the appropriate addition. If we consider cyanidation, carbon and carbonaceous matters are responsible for a phenomenon called pre-robbing. This phenomenon is produced during ore processing when this gangue takes gold from the pregnant cyanide solution during leaching. When this situation occurs, gold recovery is extremely low. The solution to this problem is not simple and a pre-treatment before cyanidation is necessary.
Quartz and iron oxides are responsible of gold losses into flotation tails. When gold is present as fine inclusions or encapsulated, the fineness of primary grind is not enough to liberate gold particles. This is a special case of many polymetallic operations such as copper-zinc or lead-zinc that are not able to recover gold into the copper or lead concentrate.


Gold and pyrite locked by quartz Gold locked by gangue
Mineralogical Studies
These studies are necessary to determine gold particle size and mineral surfaces. Measurement of gold particles plays an important role in the test work program, design and operative conditions required to get high gold recoveries. In this way, the comminution circuit is an important part of the design. The behavior of ore particles in concentration and leaching processes is strongly dependent on their size.
It will be important to determine the degree of liberation of gold particles, interlocks, associations with sulphides and gangue minerals. Obviously, optical examination will let us know the type of gangue present and the quantities of carbonaceous matter into the ore. Basically the process comprises several steps: sampling, size analysis, assays, head mineralogical analysis, and products mineralogical analysis.
Sampling is a key stage in all mining project because all the samples to be selected represent the different mineralized zones of the ore body. The mineralogical program must be oriented to highlight different ore types and be combined with geological distribution and logging information. In this way the different structures or rock formations provide indication on the behavior of the ore. If the samples will be selected from a drilling program, the different holes give information about the hardness, abrasion, and mineralogy of some areas. By studying the faults and structures is possible to get information on the different ore classes and the degree of oxidation, which must be considered in the mining plan. All these criteria help to take the decision on how to take the samples and perform composites.
It is important to study the material with low mineralization. Typically this material is considered as waste material and dilution material. In some cases, some of these materials can have a negative effect on gold recovery due to the potential presence of carbonaceous compounds or organic matter. Then is important to take some samples and include them in the mineralogical study. Some mineralized zones have higher gold content than other zones and the dilution material will be mixed to balance the gold content to be sent to the operation.
Size analysis of the samples collected is appropriate to have a primary idea of the distribution of different ore types under the same sample preparation procedure. This means, that all the samples must be crushed and split by using the same methodology so that the generation of coarse and fine material can be produced under the same conditions. For example, if one sample was crushed in three stages, all the samples have to be crushed under the same crushing parameters. Many times, the production of fine material is overlooked and the lack of knowledge on sample preparation is an issue to be solved with the right work procedure. At the end of this stage all the sample must be 100% minus 6 mesh.
An estimated weight of approximately 1kg must be obtained by coning and quartering processes. The material is split in three parts so that they can be used for size analysis, assays, and the last one for mineralogical studies.
The rot-tap or other mechanized screen device needs 200 to 300 g to perform a particles size distribution. It is important to study the fraction below 38 µm due to some losses are associated to fine particles. Before performing dry screening, it is necessary to deslime at 38 µm. The regular range of sizes includes 2360, 1700, 1180, 850, 600, 425, 300, 212, 150, 106, 75, 53 and 38 µm.
| Mesh | µm | Weight, % | ||
| Individual | Retained | Cumulative | ||
| +10 | 1700 | 0.0 | 0.0 | 100.00 |
| +14 | 1180 | 5.36 | 5.36 | 94.64 |
| +20 | 850 | 10.58 | 15.94 | 84.06 |
| +28 | 600 | 13.43 | 29.38 | 70.62 |
| +35 | 425 | 10.78 | 40.16 | 59.84 |
| +48 | 300 | 10.22 | 50.37 | 49.63 |
| +65 | 212 | 9.53 | 59.90 | 40.10 |
| +100 | 150 | 7.35 | 67.25 | 32.75 |
| +150 | 106 | 6.24 | 73.49 | 26.51 |
| +200 | 75 | 5.22 | 78.71 | 21.29 |
| +270 | 53 | 5.06 | 83.78 | 16.22 |
| +400 | 38 | 3.04 | 86.82 | 13.18 |
| -400 | 13.18 | 100.00 | 0.00 | |
| Total | 100.00 | |||
Particle size distribution
Chemical analysis of the samples are carried out to know the concentration of several metals such as gold, silver, iron, copper, sequential copper, zinc, mercury and presence of deleterious elements such as aluminum, silicon, sulfur, magnesium and insoluble material. Samples show variable composition of valuable and non-valuable minerals and the metallurgist must be able to establish a correlation between the samples. Sometimes this step is not easy and the analysis must be repeated several times until get an acceptable conclusion. For example, if we have a sample with interesting contents of copper and gold, the results of sequential copper assays will help us to detect the degree of oxidation and the presence of secondary and primary copper sulphides. Low gold (0.5-2.0 g/t) content and high degree of oxidation (e.g. 0.5-1.0% total copper and 0.2-0.5% copper soluble in sulphuric acid) indicate a potential problem for cyanidation. Analyses for gold and silver are shown in next table
| Head Assay g/t | |
| Au | Ag |
| 10.2 | 140 |
Head Analysis for precious metals
Multi-element head analysis can be done by ICP in order to know metal distribution in the sample. Values higher than the range of detection must analyzed by other techniques such as titration.
| Element | Range of detection | Assay |
| Ag, ppm | 0.2-100 | >100 |
| Al, % | 0.01-15 | 8.3 |
| As, ppm | 3-10000 | 1200 |
| Ba, ppm | 1-10000 | 845 |
| Be, ppm | 0.5-10000 | 0.1 |
| Bi, ppm | 5-10000 | 56 |
| Ca, % | 0.01-15 | 1.9 |
| Cd, ppm | 1-10000 | 14 |
| Co, ppm | 1-10000 | 55 |
| Cr, ppm | 1-10000 | 18 |
| Cu, ppm | 0.5-10000 | 9965 |
| Fe, % | 0.01-15 | 8.3 |
| Ga, ppm | 10-10000 | <10 |
| K, % | 0.01-15 | 2.3 |
| La, ppm | 0.5-10000 | 10 |
| Mg, % | 0.01-15 | 1.3 |
| Mn, ppm | 2-10000 | 7805 |
| Mo, ppm | 1-10000 | 19 |
| Na, % | 0.01-15 | 0.67 |
| Nb, ppm | 1-10000 | <1 |
| Ni, ppm | 1-10000 | 19 |
| P, % | 0.01-15 | 0.12 |
| Pb, ppm | 2-10000 | 3956 |
| S, % | 0.01-10 | 0.67 |
| Sb, ppm | 5-10000 | 95 |
| Sc, ppm | 0.5-10000 | 15.8 |
| Sn, ppm | 10-10000 | 11 |
| Sr, ppm | 0.5-5000 | 140 |
| Ti, % | 0.01-15 | 0.26 |
| Tl, ppm | 2-10000 | <2 |
| V, ppm | 2-10000 | 221 |
| W, ppm | 10-10000 | 40 |
| Y, ppm | 0.5-10000 | 19 |
| Zn, ppm | 0.5-10000 | 486 |
| Zr, ppm | 0.5-10000 | 16.1 |
Multi-element head assay
Mineralogical examination of the samples indicates results are not the same for all samples. For example one sample may be characterized by low or high grade iron ores, and the phases of minerals present in order of abundance are could be pyrite, iron oxides, iron hydroxides, silicates and traces of sulphides. Oxide iron minerals are typically hematite, goethite and limonite. Gangue minerals are detected by ore petrography, X-Ray Diffraction and Scan Electron Microprobe (SEM) analysis. The most common gangue minerals are aluminous and siliceous minerals, clays (kaolinite, illite), and quartz. When the samples are not characterized for having high level of sulphides, the trace of sulphide phases may be identified by ore petrography. The main iron sulphides mineral to be found are pyrite, arsenopyrite, pyrrhotite, and marcasite.
Detailed microscopic study by transmitted and reflected light may be done to delineate various phases, arrangement, shape and size for optimum liberation. Gold carrier minerals are in disseminated form, and sometimes they can be enclosed in a matrix of oxidized iron minerals such as hematite and goethite. In such cases liberation of gold minerals from the mass is difficult and very fine grinding is required for separation of gangue from the associated ore.
Hematite is an iron mineral present in varying types, sizes and shapes from 10-50 microns to 300-900 microns grains of acicular, lath shaped, equant, crystalline to irregular shapes. These minerals may exhibit microbanding, microplaty, and reticulate textures. In some cases, annealing textures and metamorphic ball textures could be observed in iron oxides minerals.
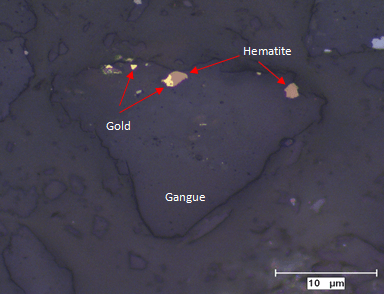
Gold associated with hematite
Quantitative Evaluation of Material by Scanning Electron Microscopy (QemScan) is a good option for mineralogical studies in order to get complete information of the minerals present in the samples. This technique employs back scattered electron signal intensity and energy dispersive X –ray signal at each point to be measured. The system is configured to measure mineralogical variability considering the metallic content at micro scale.
Once the particle size distribution is obtained, the fractions will be submitted for chemical analysis and mineralogical analysis by QemScan. Subsamples of each fraction of each material are used to prepare polished sections in graphite. Replicated sections are analyzed so that the mineralogist can obtain detailed information for speciation and distribution studies. Typically, these studies comprise around of 100,000 points per fraction.
QemScan studies are divided in three categories, bulk mineralogical analysis (BMA), particle mineral analysis (PMA) and specific mineral search (SMS). The election of the study is based on level of studied required and the type of sample (head, concentrate, tail). The first one employs a liner intercept method and generates a strong determination of the mineralogy indicating minerals, proportion and average grain size. The second category provides a particle mapping of measurements that provides entire information of the mineralogy, liberation, association and grain size distribution. The last one is focused on mapping specific particles that contain important elements. In this way is possible to get complete information of the mineral of importance.
| Mineral Distribution | Mineral distribution at different sizes | |||||
| % | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
| Sample particle size | 212 | 150 | 150 | 106 | 212 | 106 |
| Chalcopyrite | 1.32 | 0.67 | 0.72 | 0.85 | 0.76 | 0.95 |
| Arsenopyrite | 0.12 | 0.35 | 0.23 | 0.98 | 0.57 | 1.14 |
| Marcasite | 0.15 | 1.24 | 0.97 | 2.23 | 1.29 | 0.56 |
| Pyrite | 0.21 | 2.15 | 1.34 | 3.34 | 1.89 | 6.89 |
| Quartz | 83.2 | 87.32 | 77.98 | 90.23 | 85.46 | 79.34 |
| K-feldspar | 0.45 | 1.23 | 2.45 | 0.23 | 1.56 | 0.98 |
| Plagioclase | 1.32 | 0.41 | 1.18 | 0.26 | 0.99 | 0.67 |
| Sericite | 2.13 | 0.86 | 1.78 | 0.34 | 4.32 | 1.14 |
| Amphiboles | 0.45 | 0.34 | 0.98 | 0.11 | 1.14 | 0.45 |
| Chlorites | 1.56 | 0.72 | 1.23 | 0.12 | 0.67 | 0.41 |
| Tl-Fe oxides | 2.56 | 0.89 | 1.67 | 0.45 | 0.43 | 0.87 |
| Calcite | 1.14 | 0.95 | 2.69 | 0.23 | 0.12 | 3.36 |
| Gypsum | 2.15 | 0.91 | 3.56 | 0.24 | 0.54 | 2.14 |
| Apatite | 3.24 | 1.96 | 3.22 | 0.39 | 0.26 | 1.10 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Particle size distribution
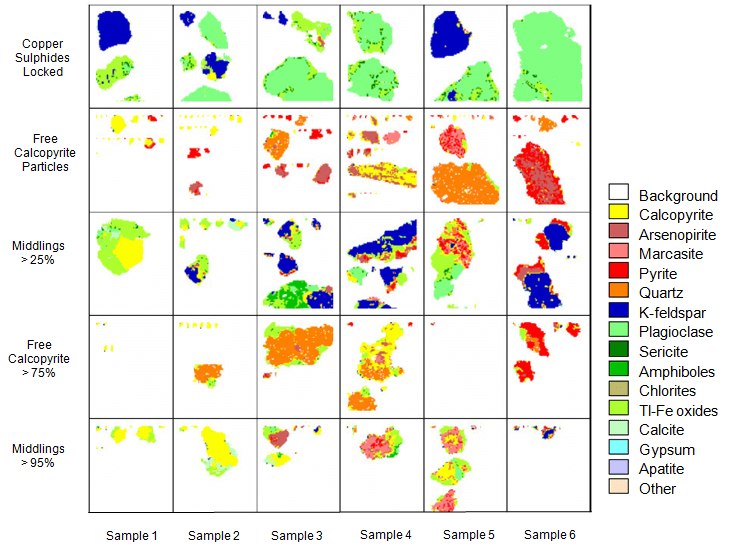
Gold-Copper Ore Mineral liberation
There are some gold deposits with variable degree of oxidation, but basically the ore can be classified in two categories, oxidized and non-oxidized. Then, both composites must be studied in order to assess their mineralogical properties are possible metallurgical behavior. Next tables show an example of some results that could be found.
Oxidized material
| Ore | % | Gangue | % | Oxides | % |
| Electrum | Traces | Quartz | 45 | Hematite | 35 |
| Pyrite | 2 | Calcite | 10 | Goethite | 0.5 |
| Chalcopyrite | Traces | Clays | 1 | Magnetite | 0.1 |
| Sphalerite | Traces | Gypsum | 0.5 | Limonite | Traces |
Non-oxidized material
| Ore | % | Gangue | % | Oxides | % |
| Electrum | Traces | Quartz | 45 | Hematite | 5 |
| Pyrite | 35 | Calcite | Traces | Goethite | Traces |
| Chalcopyrite | 0.1 | Clays | Traces | Magnetite | Traces |
| Sphalerite | Traces | Gypsum | Traces | Limonite | Traces |
The presence of cyanide and oxygen consumers such as iron oxides and sulphides indicate the leaching process will be influenced by free cyanide, cyanide dosage and dissolved oxygen into the slurry. The presence of clays may affect the leaching process due to the viscosity and pulp rheology is modified and these parameters are related to the oxygen transference. It is must be mentioned that arsenopyrite is an oxygen consumer and part of the oxygen will be used during the redox process. The second problematic sulphide is pyrite.
According to the tables, the presence of electrum is scarce in relation to the other minerals, and its recovery is influenced by the possible association with other minerals. Sometime, when the gold content is extremely low will be necessary to pre-concentrate the material before performing any mineralogical study. It is important to mention that gold grains until 90-100 microns indicate that the comminution circuit must produce a K80 of around 90 microns in order to get all gold particles liberated and the leaching process can be performed successfully, otherwise, the process will give low gold recoveries. Other aspect very interesting is the distribution of gold particles in the fine and coarser fractions in order to estimate the potential losses of any gravity process. For example if the fraction minus 38 microns contains most gold (e.g. 60 or more), gold particles are very fine.
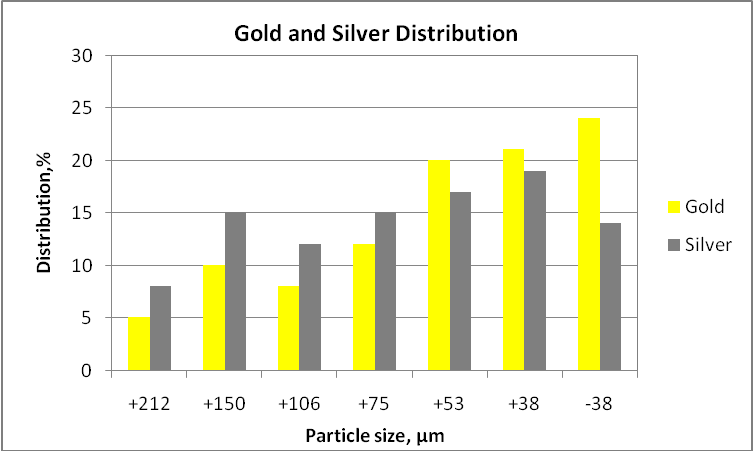
Gold and silver distribution size by size
There is other important technique employed to study the mineral size distribution and the occurrence of gold into the samples. This technique is called Mineral Liberation Analyzer (MLA), which is supported by scanning electron microscope and energy dispersive X-ray to study the polished section at different fraction sizes. Besides, back scattered electron images generated during the study are employed to analyze grain boundaries and X-rays are used to study grain minerals. Basically, mineral liberation analyzer provides mineralogy distribution, grain size, surface areas and mineralogical association.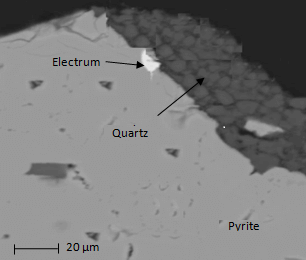
Electrum in the border between quartz and pyrite
Gold Flotation results are supported by mineralogical studies, which have been used to gain an understanding of the mineralogical factors affecting plant performance, and this has been useful in the different steps of the project such as commissioning or optimization plant results. The objectives of the mineralogical study must be focused on understanding gold ore mineralogy and establishing relationships between gold particles floatability and mineralogy, and to study bulk sample mineralogy in order to study the effect of assemblage and liberation of gangue minerals in the concentrate.
Flotation circuits have different configurations, but in general the circuit consists of crushing and grinding with SAG milling or not. Nowadays SAG mill are the favorite for flotation plants. The grinding product is sent to the flotation circuit which consists of rougher and scavenger flotation and the concentrate is reground or not according to the plant type. If the plant includes a regrinding stage, vertimills or isamills are employed to liberate particles. The reground product is sent to the cleaner circuit. The cleaner circuit consists of two or three stages and one cleaner-scavenger stage. If the ore present high contents of slimes such as talc or clays, column cells are employed to clean the concentrate. The presence of undesirable minerals is attack by flotation machines and specific flotation reagents to depress these materials. Probably, carboxy methyl cellulose has been the most effective reagent. Under this consideration will explain some problems in gold flotation plants.
Mineralogical studies were carried out on head samples taken during 12 hours. The gold content reported was 6 g/t and the mineralogy can be very different if the mine sends a different material. The mineral species to be found can be free gold, pyrite or tellurides. The different minerals have an effect on gold floatability and its recovery and they are influence by the gangue present in the ore. The particle size are variable such as 1 µm to 20 µm. The frequency, area and volume distribution of these grains indicate liberation, inclusion and possible formation of grain clustering. Gold grains less than one micron and associated with gangue minerals are potential losses.
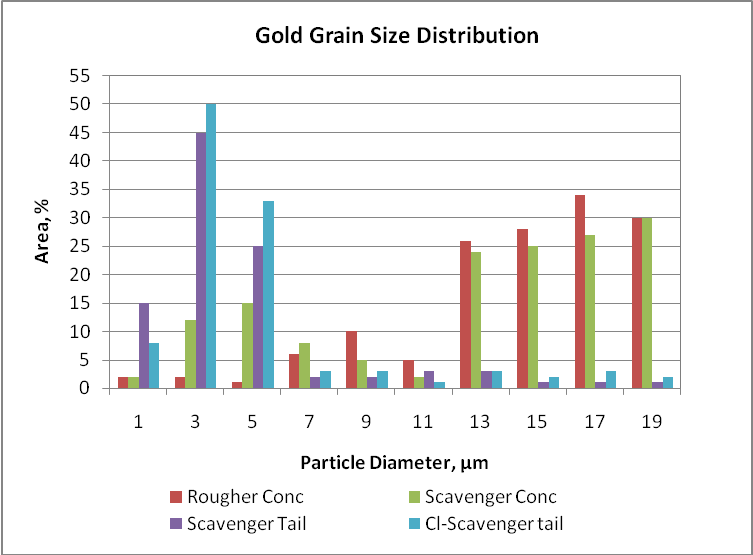
Gold size distribution in different flotation products
According to the graph, rougher and scavenger concentrates contents coarser gold particles than scavenger and cleaner-scavenger tails. It notorious the high proportion of fine particles in tails due to slow flotation or interlocks. Coarser particles have high flotation rate and they are reported in small proportion in scavenger and cleaner-scavenger tails. Then, the difference in floatability is influenced by grain size and liberation. Gold particles in rougher and scavenger concentrates show a bimodal size distribution, with a high frequency population less than 7 µm in diameter and a second smaller population ranging from 13 to 19 µm in diameter. The finer particles, even those occurring as thick clusters, float much slower and the grain size distribution of the non-floated material is related to the slow-floating gold particles and indicates that flotation has not reached its best performance. Under these observations, gold recoveries can be improved by either regrinding or floating ultra-fine liberated particles or floating more of the interlocked particles. Then, the flotation circuit needs long residence time or modify the rheological flotation parameters in order to improve gold recovery.
