Table of Contents
At the beginning of the use of the electric furnace, for the manufacture of calcium carbide and ferro-alloys, experimental work was conducted in it upon the production of steel from iron ore. For many years steel and wrought iron have been produced directly from ore on a small scale in the forge, crucible, and reverberatory furnaces; but the difficulties attending the operation of these combustion processes have prevented their adoption to any great extent. The combustion processes which have attempted to produce a product which might be classified as a pig steel rather than pig iron, have failed largely for economic reasons rather than because of metallurgical difficulties. They were not adapted to the large units necessary to turn out a large tonnage, as is the case in the manufacture of pig iron.
The introduction of the electric furnace into metallurgy somewhat renewed the interest of metallurgists in direct processes for the manufacture of steel. This is because the electric furnace can be operated with the proper metallurgical conditions for the production of pig steel rather than pig iron, and because in a possible design of shaft furnace similar to the blast furnace there is a greater chance for the production of a larger tonnage than is possible in the case of furnaces used in combustion direct steel processes. The early experiments of Stassano showed possibilities for a direct steel process in an electric furnace of the hearth type, which, however, had the same objectionable features as the combustion processes—a small output and a high cost of production. Later, during the experiments on the production of pig iron in the electric furnace at Domnarfvet, Sweden, 280 tons of crude non containing from 0.95 to 3.09 per cent, carbon were produced. This showed the possibility of producing pig steel in an electric shaft furnace. Later at Trollhattan, Sweden, these results were confirmed in a larger furnace.
In this paper the writer does not advocate the production of pig steel from ore in the electric furnace as a competitor of the present method of steel production with pig iron as an intermediate step, but presents the general metallurgical side of such a process and its economical possibilities in regions where power is cheap, and fuel and reducing materials are expensive, as, for example, Sweden, California, British Columbia, and the western coast of South America. The field of the electric furnace in pig-iron manufacture is restricted to such areas, and possibly will never have great application even in favorable districts, because of the cheapness with which iron ore can be delivered from western coast deposits to furnaces upon the eastern coast of the United States on the completion of the Panama Canal. But when in these countries local demand for pig iron and steel is great enough, the electric furnace has an opportunity. In such cases, if steel is the desired final product, it seems more feasible and cheaper to produce as pure a product as possible—i.e., pig steel—for any subsequent refining operations, rather than pig iron. This would prove to be the case for two reasons:
- As has been found to be the case at Heroult, Cal., Domnarfvet and Trollhattan, pig steel can be produced more cheaply in the electric shaft furnace than pig iron, because of the greater output per unit of electrical energy consumed.
- Owing to the lower percentage of impurities in pig steel, 1 to 3 per cent., instead of 4 to 8 per cent, in pig non, a greater output can be obtained from the final refining furnace at a lower cost.
Electric Furnace Production of Pig Steel
In his discussion of electric-furnace production of pig iron and pig steel before the Engineers’ Society of Western Pennsylvania, Dr. J. W. Richards defines pig steel as “a metal with 2.2 per cent, or less of carbon, a very small amount of silicon and manganese, low in sulphur and phosphorus, and made directly from iron ore in the electric pig iron furnace.” That is, in chemical composition it is steel. If it resembles steel in chemical composition, there is no reason why it should not possess the same physical qualities as steel, when subjected to the same conditions of pouring and heat treatment as a combustion-furnace steel of the same composition with which it may be compared. Or it should conform to the definition of steel as given by Stoughton: “iron which is malleable at least in some one range of temperature, and in addition is either (a) cast into an initially malleable mass; or (b) is capable of hardening greatly by sudden cooling; or (c) is both so cast and so capable of hardening.”
A process to produce efficiently a metal conforming to these requirements should satisfy the following conditions:
- As the product in order to be a steel must not contain over 2.2 per cent, carbon, it should be possible not only to keep the percentage of carbon in the metal below that amount, but it should also be possible to regulate the carbon within reasonable limits; i.e., control the composition of the metal.
- The product should contain percentages of silicon, phosphorus, and sulphur either below the limit set by consumers of Bessemer and open- hearth steels, or at least low enough not to require prolonged refining in another furnace.
- The loss of iron in the slag should not be excessive.
- The furnace used must be adapted to continuous operation and the production of a large tonnage.
- By the process it should be possible to produce pig steel at a greater profit than by existing methods.
Considering the possibility of the use of the electric furnace to satisfy these requirements:
- As it is not necessary to introduce carbon for fuel in the electric furnace, it is not necessary to have excess carbon in the charge beyond that needed for reduction, therefore no difficulty should be experienced in keeping the carbon of the product below 2.2 per cent.; and it should be possible to regulate the carbon within reasonable limits.
- The temperature of the electric furnace can be regulated by the power input, so that very basic slags can be used, which should result in slagging of silicon, phosphorus, and sulphur.
- While the loss of iron in the slag with an electric furnace running on pig steel would probably be greater than in the case of pig iron, owing to the weaker reducing atmosphere of the pig-steel furnace, it should not be so excessive as to prohibit the use of such a process under favorable economic conditions.
- The electric shaft furnace has been proved to be easily adapted to continuous operation for pig-iron production, and should operate more uniformly on pig steel than on pig iron because there is not the tendency for carbon to accumulate in the furnace.
- In localities where electric-furnace pig iron can be produced at a profit, pig steel for further refining could be made at a profit if the market demand was for steel.

https://www.interstahl.com/en/steel-wiki/pig-iron-production.html
The direct production of steel from iron ore in the electric furnace has never passed beyond the laboratory stage. Just as pig iron can be produced from iron ore in the electric furnace, so can pig steel be produced by a close regulation of the carbon content of the charge. Sufficient carbon is provided for reduction of the ore, but not enough to combine to any appreciable extent with the product. This of course cannot be done in the blast furnace because of the excess carbon present for fuel. The ore is reduced to pure iron, but this combines at once with any free carbon present. In the electric-furnace the electrical energy, does the heating, while carbon simply performs the chemical function of reduction. The reduction of the ore to pig steel is comparatively simple, but some difficulty has been experienced in preventing a high loss of iron in the slag, due to the low carbon content of the charge. Also, as in all direct steel processes, the commercial success of a similar electric furnace process is yet to be determined by operations conducted on a large scale.
Some experimental work performed by the writer in 1911 under a Carnegie Research Scholarship of the Iron and Steel Institute of Great Britain, and the work of others, serve to show the metallurgical possibilities of the electric furnace for the production of pig steel from ore, and whether the process satisfies the five stated requirements.
Making Pig Iron by Electric Furnace
Five groups of experiments were made on the electric-furnace production of pig steel, arranged as below:
Group I. A series in which both limestone and coke were varied in the charge.
Group II. A series in which the amount of coke in the charge was varied, other components remaining constant.
Group III. A series in which the amount of limestone in the charge was varied, other components remaining constant.
Group IV. A series in which a part of the limestone was replaced by varying amount of fluorspar, other components remaining constant.
Group V. An experiment in which the furnace was operated continuously rather than intermittently, as in the other experiments.
Because of the impossibility of tapping a small furnace cleanly after each experiment, it seemed advisable, in order to more closely represent large-scale working, to calculate the theoretical weight of the pig steel present from the loss of iron in the slag and the analyses of the pig steels tapped, considering all iron available which did not pass into the slag. The results of these calculations were shown to check closely with the total amount of metal obtained during all of the experiments, including the amount cleaned out of the furnace at the end. Of the total calculated 89.4 per cent, was tapped, and 93.6 per cent, was reduced according to calculations.
These experiments were performed in a furnace, lined with magnesite, having two upper vertical graphite electrodes connected in parallel, and a conducting hearth of iron rods imbedded in magnesite, the hearth and the graphite electrodes being connected in series. The interior dimensions of the crucible were: length, 9 in.; width at the bottom, 4 in.; depth, 12 in. This furnace took from 10 to 12 kw. of single-phase current at 30 to 50 volts, and held about 25 lb. of cold charge.
Analyses of the raw materials used are given in Table I.
Regulation of Carbon
The pig steels produced varied in composition with the coke in the charge from a low-carbon to a high-carbon pig steel. With one exception, they all contained less than 1.75 per cent. This exception was a white iron containing 2.25 per cent, carbon. The percentage of manganese was uniform, with an average of 0.11 per cent. The silicon varied to a considerable extent with the basicity of the slag, but could be kept below 0.15 per cent, without difficulty The average percentage of silicon in the pig steel was 0.12 per cent. The percentages of phosphorus and sulphur in the pig steels varied under different conditions. The loss of iron in the slag was not excessive. From the results obtained, it seems that the regulation of carbon in a pig steel produced from ore in the electric furnace is possible within rather close limits.
As shown in Table IV, the percentage of carbon in the pig steel gradually increased with the increase of carbon in the charge, up to about 1.50 per cent, in the pig steel, above which the amount of carbon absorbed does not increase as rapidly as the increase of carbon in the charge. To determine whether there is any definite relation between the carbon charged and the carbon entering the steel, the results of some of the experiments have been figured to pounds of carbon charged per pound of pig steel reduced, and from this the amount of carbon entering the metal has been reducted in each case, giving the pounds of carbon actually used per pound of pig steel reduced for reduction only, Table II.
Variation of Limestone and Coke in the Charge.—In Group I (Table III) the results of experiments Nos. 3, 4, and 5 indicate a constant relation between the carbon charged and that entering the pig steel. The pounds of carbon required for reduction only per pound of pig steel reduced varied from 0.2781 to 0.2762 lb., averaging 0.2771 lb. A higher figure was obtained than from the results of Group II, Table IV, which was probably due to the charge being more basic in the former case.
Variation of Coke in the Charge.—In Group II (Table IV) the lime charged was constant, although, in calculating it to pounds of pig steel reduced, it appears to be variable because of variation in the amount of pig steel reduced. From this group a pig steel containing from 0.48 to 1.57 per cent, carbon, there appears to be a constant amount of carbon required for reduction only, any added above this amount serving to carburize the pig steel. This amount varies between 0.2683 and 0.2704 lb., averaging 0.2694 lb. That is, if 0.2694 lb. of carbon were charged per pound of pig steel reduced, theoretically the product would be free from carbon. If 0.2794 lb. of carbon were used in the charge the product would contain 1.00 per cent, carbon. Above 1.57 per cent, carbon in the pig steel the excess of carbon in the charge does not seem to carburize the pig steel at the same rate.
Variation of Limestone in the Charge.—From the results of Group III (Table V), in which lime in the charge was varied, other factors remaining constant, with the increase of lime in the charge seemed to be
a gradual increase of carbon required for reduction, and a corresponding decrease of carbon in the pig steel. This is probably due to the formation of calcium carbide with the increased percentage of lime present.
In Group I, experiments 3, 4 and 5, with increased basicity of the charge the carbon in the pig steel decreases, while that used for reduction increases as in Group III. The decrease does not appear so marked with the more basic charge used in Group I. The carbon content of the charge was considerably less than that of Group III.
Variation of Fluorspar in the Charge.—In Group IV (Table VI) the increase of fluorspar in the charge causes a marked increase of carbon in the pig steel and a corresponding decrease of carbon used for reduction, due probably to excessive thinning of the slag.
The experiments of Neveu and Arnou in 1910 in an intermittent electric furnace of the hearth type showed the ease with which pig steel could be made, using coke, anthracite, or charcoal as a reducing material. Hematite, magnetite, and siderite were reduced to pig steel, when charged in either a coarsely divided or a pulverent state.
Trouble was experienced at the Hardanger, Norway, electric pig-iron plant with coke as a reducing material. The failure of this plant
has been assigned to the use of coke, because coke has a higher electrical conductivity than, charcoal and hence not as much heat is generated by the resistance of the charge to the passage of the electric current. For this reason, trouble might be experienced, with a shaft furnace using coke as a reducing agent in the production of pig steel, but the problem will undoubtedly be solved eventually. The writer visited the Hardanger plant a few months before it was closed, and from observation of existing conditions does not believe the failure of the enterprise was entirely due to use of coke.
J. Crawford says that, at Heroult, while difficulty was experienced with coke alone as a reducing material, “by adopting certain precautions in crushing the stock and feeding the same into the furnace I have operated on a mixture of 60 per cent, coke and 40 per cent, charcoal with a very fair degree of furnace efficiency.”
He further remarks: “I believe that many of our coals which make a poor metallurgical coke for blast furnace use on account of their low crushing strength might be found to make a satisfactory fuel for electric furnace use.”
The experiments of Neveu and Arnou do not show anything regarding the regulation of carbon in the product beyond that pig steels containing from 0.08 to 1.25 per cent, carbon were produced.
The results at both Domnarfvet and Trollhattan indicate that pig steel can be made directly from ore in the electric shaft furnace. In these runs no attempt was made to produce pig steel, but the product contained from 1 to 3 per cent, carbon, as before stated. While it would undoubtedly be more difficult to regulate the carbon of the product in a shaft furnace operated continuously than in an intermittent hearth furnace, it could be done, but the regulation would not be so close.
Hence it may be stated that:
- In producing pig steel containing from 0.25 to 1.50 per cent, carbon for any particular furnace and basicity of charge, the carbon content of the pig steel can be regulated by varying the carbon in the charge.
- An increase or decrease of lime in the charge causes a corresponding decrease or increase of carbon in the pig steel.
- The use of fluorspar in excessive quantities causes a marked increase in the carburization of the pig steel.
- The experiments of Neveu and Arnou indicate that the carbon of reduction may be supplied from coke, anthracite coal, or charcoal, and the charge may be fine, coarse, or briquetted.
- The work at Domnarfvet and Trollhattan shows the possibility of keeping the carbon of the product below 2.2 per cent, in an electric shaft furnace.
Pig Iron Impurities
Silicon.—The results of Group II show that with an increased amount of coke in the charge there is a gradual, although not excessive, increase of silicon passing into the pig steel. The increased basicity of the charge, Group. III., lowers the percentage of silicon in the pig steel. Increased charging of fluorspar seems to cause a decrease of silicon in the pig steel, Group IV. In general, the experiments show that the percentage of silicon in the pig steel can be kept at a low figure if desired, and that its regulation does not seem difficult.
This conclusion is corroborated by earlier investigators. In his first experiments, with an ore containing 2.79 per cent. SiO2 and very pure charcoal as a reducing agent, Stassano obtained products with from a trace to 0.048 per cent, silicon. His second experiments, with ore containing 17.15 per cent. SiO2, resulted in pig steel with from 0.03 to 0.22 per. cent, silicon. Brown and Lathe, using hematite with 2.23 per cent. SiO2, had from a trace to 0.24 per cent, silicon in the pig steels produced. The work of Neveu and Arnou on hematite, magnetite, and siderite ores of from 0.6 to 8.96 per cent. SiO2 gave products containing 0.02 to 0.19 per cent, silicon. Considering that the ore used contained 9.25 per cent. Si02 and,the coke 13.62 per cent. SiO2, the results of the writer, an average of 0.15 per cent, silicon in the product, show that no difficulty would be experienced with poor coke and impure ores.
Phosphorus.—Although the ore containedra high percentage of phosphorus, 0.124 per cent., no difficulty was experienced in keeping the percentage in the metal low. In the 20 experiments from 51.7 to 95.92 per cent, of the total phosphorus charged was slagged, with an average of 77.25 per cent. (See Table-VII.)
In Group II, the gradual increase of coke in the charge caused an increase of phosphorus in the pig steel, because of the failure of the phosphorus to pass into the slag as calcium phosphate under the more reducing conditions of the operation. Increased lime in the charge, Group III, seems to result in an increased percentage of phosphorus in the pig steel. This was probably caused by the more basic slag being too thick for intimate contact with the steel bath during the agitation caused by the passage of the electric current through it.
Fluorspar, Group IV, causes a slight decrease of the percentage of phosphorus in the steel when used in small quantity, but when charged in excess it caused the percentage of phosphorus in the pig steel to increase. The slight thinning of the slag seemed to increase the slagging of the phosphorus. Continuous operation of the furnace did not cause increased phosphorus in the pig steel, as 89.3 per cent, of the phosphorus charged was slagged, and the pig steel produced contained 0.031 per cent, phosphorus.
These results agree with those of other investigators. Stassano in his first experiments used an ore containing 0.058 per cent, phosphorus, which resulted in pig steels with 0.005 to 0.024 per cent. In his second series the ore contained 0.15 per cent, phosphorus and the pig steel 0.01 to 0.10 per cent. Brown and Lathe, with an ore containing 1 per cent, phosphorus, obtained products with 0.031 to 0.49 per cent, phosphorus, but did not reduce the phosphorus in the pig steel to below 0.20 per cent, without excessive loss of iron in the slag. As the carbon content of the change was decreased the percentage of phosphorus in the pig steel decreased, but at a 0.091 per cent, carbon pig steel the FeO in the slag was, 26.94 per cent, and phosphorus in the pig steel 0.031 per cent., whereas with 0.54 per cent, carbon the pig steel contained 0.20 per cent, phosphorus and the slag 3.6 per cent. FeO. In the work of Neveu and Arnou, with ores of 0.02 to 0.052 per cent, phosphorus content and pig-steel products of from 0.08 to 1.25 per cent, carbon, the phosphorus in the metal was reported as a trace.
Yngstrom asserts in his report on the Domnarfvet experiments that the phosphorus goes almost entirely to the iron. Considering the con-
ditions of the runs this is to be expected, because, with the carbon necessary to produce a pig iron, the atmosphere of the furnace was too reducing to allow oxidation of the phosphorus. Also the slags used in some runs were very acid, as high as 41.1 per cent. SiO2. Leffler and Nystrom in their 1912 report on the Trollhattan furnace show results somewhat similar to those at Domnarfvet. From ores containing 0.002 to 0.055 per cent, phosphorus, metal was produced which contained 2.68 to 3.85 per cent, carbon and 0.013 to 0.045 per cent, phosphorus, with acid slags of from 36.54 to 45.15 per cent. SiO2.
The conclusions drawn from these results are:
- Increased carbon in the charge causes an increase of the percentage of phosphorus in the pig steel, because of the stronger reducing conditions.
- Increased basicity of the slag does not cause the slagging of more phosphorus if the slag is very, thick.
- If a small amount of fluorspar is added to thin the basic slag, the slagging of the phosphorus is assisted.
- Continuous operation of the furnace does not increase phosphorus in the pig steel.
- Owing to the more oxidizing conditions in the electric furnace when pig steel is being made rather than pig iron, a greater percentage of the phosphorus is slagged.
Sulphur.—The hematite used in the experiments contained 0.14 per cent, sulphur and the coke 0.54 per cent. Contrary to expectations, no difficulty was experienced in concentrating the sulphur in the slag. From 66.6 to 96.8 per cent, of the total sulphur in the charge was slagged, with an average of 87.28 per cent.
In Group II, the sulphur acted contrary to the phosphorus with the increased reducing atmosphere, being easily slagged, owing to the better conditions for the formation of calcium sulphide. In Group III, the increase of lime in the charge caused a marked increase in the amount of sulphur passing into the slag. The coke in the charge was low in amount, so that some of the sulphur probably passed into the slag as calcium sulphate. From Group IV, it seems that a small amount of fluorspar in the charge aids in the elimination of sulphur from the pig steel, but an excess causes it to pass into the pig steel. As in the case of phosphorus, more sulphur passed into the slag when the latter was fluid. Continuous operation of the furnace did not cause increased sulphur in the pig steel, as 96.8 per cent, of the sulphur charged was slagged, and the pig steel produced contained 0.048 per cent, sulphur.
In Stassano’s first experiments the difficulty of elimination of sulphur was shown, as, from ore with 0.058 per cent, sulphur, the pig steels produced contains 0.046 to 0.073 per cent, sulphur. From the writer’s experiments it appears that the slag must be very fluid and basic if sulphur is to be slagged in the making of pig steel, because of the weaker reducing atmosphere of the furnace. The slags used by Stassano were probably not basic enough. In his second series, from ore with 0.12 per cent, sulphur he obtained pig steels containing from 0.04 to 0.07 per cent, sulphur. Brown and Lathe, operating a furnace continuously, had difficulty in slagging sulphur. From ore containing 1 per cent, sulphur pig steel was produced which contained from 0.54 to 1.04 per cent, sulphur. This was probably due to either the furnace not being hot enough, or the slag, although basic, not being fluid enough. In some previous experiments by the writer these factors were found to be the cause of high content of phosphorus and sulphur in the pig steels produced. An illustration of the power of the electric furnace to slag sulphur with a heavy lime slag, if the slag is kept fluid by use of fluorspar, is shown in an experiment made by the writer for the production of ferro-molybdenum from molybdenite concentrate. There was 30 per cent, sulphur in the ore, while the ferro-molybdenum contained 0.19 per cent, sulphur, 0.73- per cent, carbon, and 50.55 per cent, molybdenum. Neveu and Arnou produced pig steel with 0.012 to 0.16 per cent, sulphur from ore containing 0.023 to 0.40 per cent, sulphur. The Domnarfvet runs show that from a charge containing 0.50 per cent, sulphur a pig iron and occasionally a high-carbon pig steel was made containing less than 0.005 per cent, sulphur. Similar results were obtained at Trollhattan. The conclusions formed are:
- An increased reducing atmosphere aids in the passage of sulphur into the slag.
- Increased basicity of the slag causes more of the sulphur to be slagged.
- By thinning the slag a small amount of fluorspar assists in sulphur elimination from the pig steel, but an excess has the contrary effect.
- Continuous operation of the furnace does not cause increased sulphur in the pig steel.
- With a fluid basic slag, sulphur can be slagged readily in the electric furnace production of pig steel in both a continuous and an intermittent operation.
Loss of Iron in the Slag
The ferrous oxide in the slag was affected by the degree to which the operating conditions were reducing. With increase of coke in the charge the percentage of ferrous oxide in the slag was decreased, but was little affected by the variation of basicity of slag. The extremely high results in experiments Nos. 1 and 2 are due to the carbon in the charge being made slightly less in amount than the calculated, because a very low carbon pig steel was desired. The high loss of iron in the slag of the continuous run was caused by tappings of slag when unreduced ore was low in the furnace. Some of the unreduced ore passed into the slag. In the operation of such a small furnace continuously this would be a natural result, but it should not occur with a large furnace. The slags of all the experiments contained an average of 7.47 per cent, ferrous oxide. The average loss of iron in the slags was 6.09 per cent.
The results of the writer check closely the results of Neveu and Arnou, in which for a normal run the slag contained less than 8 per cent, ferrous oxide. Brown and Lathe had 3.6 per cent. FeO in the slag tapped with a 0.54 per cent, carbon pig steel, but had 26.94 per cent, ferrous oxide with a 0.091 per cent, carbon pig steel. In the writer’s experiments, with a 0.08 per cent, carbon pig steel, there was 14.23 per cent, ferrous oxide in the slag. Evidently for pig steels containing less than 0.25 per cent, carbon there is apt to be high iron loss in the slag. At Domnarfvet, when a pig steel containing 1.70 per cent, carbon was to be tapped the slag contained 0.23 per cent. FeO, which checks closely with experiment No. 12, 1.64 per cent, carbon in the steel and 0.50 per cent, ferrous oxide in the slag.
With steady operation in a large furnace, the loss of iron in the slag should not exceed 4 per cent, of the total iron charged, depending on the amount of carbon desired in the product, but if a pig steel containing less than 0.25 per cent, carbon is produced there will probably be a greater loss of iron in the slag.
Adaptability of the Process to Continuous Operation
To produce a pig steel directly from ore in the electric furnace at a cost low enough to compete even in remote regions with imported standard steels, a process must have possibilities of large output. While in the present development the electric pig-iron furnace is small in unit compared with the blast furnace, it will undoubtedly be eventually increased much above the present capacity. Applied to the production of pig steel, the electric pig-iron furnace offers much greater possibilities for large-scale work than any other furnace yet used in direct processes. In a shaft furnace of this type the energy consumption can be kept at a lower figure than in an intermittent furnace, because of the use of gases for preheating and reduction of the charge.
An experiment was made, extending over several hours, to note the effect upon the pig steel of continuous charging and tapping rather than intermittent operation of the furnace. In addition to hematite, limestone, and coke a small quantity of fluorspar was charged. A tapping was made about the middle of the run, which resulted in the following products:
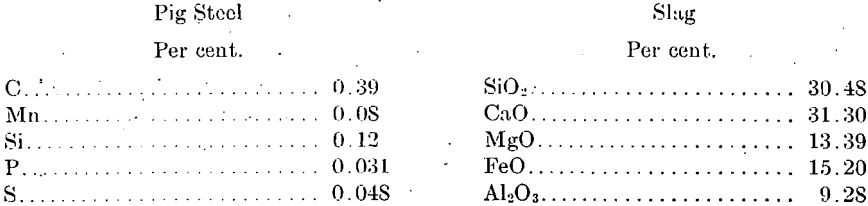
The power consumption was 3,940. kw-hr. per long ton of pig steel. From the product it may be seen that as good a grade of pig steel can be produced in an electric furnace when operated continuously as when operated intermittently.
The results of electric-furnace pig-iron manufacture in Sweden also show the feasibility of pig-steel production in the electric shaft furnace, as regards carbon and impurities in the pig steel and loss of iron in the slag.
J. Crawford remarks as a result of his experience at Heroult with an electric shaft furnace: “The matter of too little carbon gives less trouble, and if the furnace is producing low silicon and carbon iron should give none at all.” From his results it appears that it is easier to operate the shaft furnace when making pig steel than when making pig iron.
From the results available it seems that there, should be no greater difficulty in the elimination of impurities from pig steel in a continuous shaft furnace than in intermittent operation.
The following process might be used for the direct production of pig steel from ore in a continuous electric shaft furnace: The mixture of ore, flux, and reducing agent is charged continuously into an electric shaft furnace so that the shaft is full all the time. A pig steel containing less than 2.2 per cent, carbon is tapped from the furnace into a ladle for transference to an open-hearth or electric furnace if further refining is necessary, or if not, into an externally heated mixer for deoxidizing and addition of ferro-alloys. While the carbon can be regulated to a certain extent by the quantity charged, if the production of a low-carbon steel , below 0.25 per cent, carbon, is attempted by reducing the amount of carbon in the charge, there will probably be excessive loss of iron by slagging. To avoid this loss advantage can be taken of the fact that the greater part of the reduction in the electric shaft, furnace is performed by solid carbon in the upper part of the crucible. In the continuous experiment performed, it was noticed that there was a tendency for fused unreduced iron ore to work down into the bottom of the crucible. In spite of the presence of carbon in the charge sufficient to reduce the ore, when metal was tapped with the furnace full of charge the pig steel contained less carbon than the product from intermittent runs in which about the same amount of carbon was charged. For example, in experiment No. 4, 0.263 lb. of carbon was charged per pound of iron, and the pig steel contained 0.53 per cent, carbon, in comparison with 0.39 per cent, carbon in the pig steel tapped during a continuous run with 0.268 lb. of carbon per pound of iron. Experiment No. 9 shows a similar result.
This possibly may be explained by the fact that since there is only enough carbon for reduction present in the electric furnace, after the ore is reduced in the upper part of the crucible and the lower end of the shaft, there is not so great an opportunity for the iron to come in contact with the coke as is the case in blast-furnace smelting when sponge iron is carburized at the tuyeres, so that a comparatively pure product settles out in the crucible of the electric furnace. Or, it is possible that, due to the fact that in the electric furnace reduction takes place in close proximity to the pig-steel bath, some unreduced ore works down into the bath and decarburizes it. This was apparently, the case in some experiments on the production of ferro-chrome, when the percentage of carbon was considerably lower in the alloys produced by operation with the furnace shaft, full at all times than with intermittent runs. In this case reduction could not have taken place very high up in the furnace, as chromite is not reduced until a temperature of 1,185° C. is reached.
A question might arise as to ease of tapping pig steel from a shaft furnace, because pig steel has a higher melting point than pig iron. The writer had no greater difficulty in this respect with the small furnace used than is ordinarily experienced in experimental furnaces. In fact, 60 per cent, ferro-tungsten was readily tapped. In the experimental runs at Domnarfvet 1 per cent, carbon pig steels were tapped. It is probable that pig steel could be tapped from the Swedish type of electric shaft furnace as readily as pig iron, because of the concentration of heating in the crucible.
Cost of Production of Pig Iron
Cost of production of pig steel from ore in the electric furnace is, of course, the main stumbling block to the success of such a process. As yet not enough practical work has been done to give conclusive figures. All work to date has been entirely experimental, and while it has shown that the metallurgical difficulties are not impossible to overcome, little data as to actual cost is available.
It may be stated, however, that in any place where there is a market for steel, and pig iron can be made at a profit in the electric furnace, it will be more profitable in the end to make pig steel for subsequent refining in an electric furnace or open-hearth, than to produce electric-furnace pig iron with a subsequent refining to steel. This is true for two reasons:
- The power consumption has been found to be lower per ton of pig steel than per ton of pig iron, which results also in a decreased labor cost per ton, as no more labor or capital outlay is necessary to operate the plant for pig steel than for pig iron.
- As the pig steel contains a comparatively low percentage of carbon and impurities, the time necessary for refining is reduced, which results in a greater output at a lower cost.
The results of the early experiments at Domnarfvet show that the power consumption per ton of metal produced decreased as the amount of carbon charged per ton of iron was reduced.
In the latest report of the engineers at Trollhattan, it is stated that the power consumption per ton of pig iron varies in proportion to the iron content of the ore. A poor ore and pig iron high in silicon and manganese require more power than a rich ore and pig iron low in silicon and manganese. This was also found to be the case in California. The results of the writer, of course, show a high power consumption because of the small size of the furnace.
Among other expenses of pig-steel production, maintenance and capital charges would be about the same as for electric-furnace pig-iron production. The labor cost per ton of product would be a little lower for pig steel. The electrode consumption per long ton of pig iron has been reduced to 6.7 lb. It might be a little higher in making pig steel, because of there being less carbon in the charge, but it should not exceed 15 lb. per ton of pig steel.
The use of pig steel in the open-hearth was tried out at Degerfors, Sweden. It was found that pig steel produced by the electric shaft furnace was more suitable for steel making in the open-hearth than ordinary pig iron, and required less time for complete refining. Normal pig iron made in the electric furnace was found to be less suited to the production of open-hearth steel than normal blast-furnace pig iron. This shows the advantage of production of pig steel rather than pig iron in the electric furnace when steel is to be the final product.
Pig Iron
- In the electric-furnace production of pig steel from ore, carbon in the product can be kept below 2.2 per cent., and regulated to an extent by the amount of carbon charged, without resulting in excessive loss of iron in the slag or in the production of a pig steel very high in impurities, if a fair grade of ore is used.
- It is not difficult to slag the greater part of the silicon, phosphorus, and sulphur of the charge, if the furnace is hot and the slag fluid, but conditions are less favorable to the slagging of sulphur than of other impurities in the operation of an electric furnace for pig-steel production, which is, of course, contrary to experience in the manufacture of pig iron.
- The loss of iron in the slag should not be excessive unless the pig steel produced is of very low carbon content.
- From the results with the Domnarfvet, Trollhattan, and Heroult furnaces, there does not appear to be great difficulty attending the production of pig steel in an electric shaft furnace, and in fact experience has shown that there is less difficulty in the operation of the electric furnace on pig steel than on pig iron.
- At any place where there is a market demand for steel, and pig iron can be made in the electric furnace at a profit, the steel ultimately produced would be cheaper, if made by the electric reduction of iron ore to pig steel, followed by refining in another furnace if necessary, than if the product of the electric-reduction furnace was pig iron to be subsequently converted to steel in another furnace.

