Table of Contents
In this bulletin the subject of ore dressing is approached from the economic rather than from the technological standpoint, and the supporting data have been compiled with a view to their bearing upon the economic exploitation of ore deposits. Results obtained are stressed rather than the details of processes and the physics and chemistry involved. For discussions of the latter, the reader is referred to numerous publications prepared by the Metallurgical Division of the Bureau of Mines, textbooks on ore dressing, the technical journals, and publications of the technical societies.
The object of ore dressing is, broadly, to prepare the crude ore for more economical transportation and for further processing to recover the metallic contents. Although perhaps not ore dressing in the strict sense of the term, the direct extraction of the metals (especially the precious metals) from the ores also is considered here as an ore-dressing or a “milling” process. Preparation by ore dressing includes the concentration of the valuable minerals within a smaller bulk than that of the original ore by removal of worthless constituents, the separation from each other of the different valuable minerals to give separate products in each of which the minerals of one metal predominate, or the removal of constituents that have a deleterious effect in further processing and thus result in a penalty charge by the smelter.
By concentrating the metal contained in the crude ore through removal of valueless constituents, the gross weight is reduced to a fraction of that of the original ore, thus lowering the transportation cost per unit of contained metal. Many ores that could not possibly be worked if shipped in the crude state can be worked profitably if concentrated before shipment.
In commercial concentration processes some ore mineral is necessarily lost in the tailing or reject, the percentage so lost ranging between wide limits, depending on the character of the ore and the refinements of the ore-dressing methods employed. The question of how far to go with ore-dressing refinements to effect a high percentage of recovery of the valuable constituents of the ore is an economic one. Each step in the concentration of an ore adds to the cost of the operation, and unless the value of the additional mineral recovered exceeds the extra cost of recovering it, the refinement is obviously uneconomical.
Complex ores containing minerals of two or more metals frequently can be exploited economically only if the different metals are separated before smelting. For example, lead ores or concentrates are charged a penalty by the smelter if they contain more than a certain percentage of zinc; and if the zinc content cannot be reduced below a certain limit, the penalty, which increases with increase in zinc content, may be so great that it would be unprofitable to operate. In this connection it is noted that many complex western ores could not be worked profitably and were left unmined for years even though high in lead, zinc, and silver, before concentration by differential flotation was developed. By means of differential flotation it became possible to make a high-grade lead concentrate containing very little zinc and a high-grade zinc concentrate containing little lead, both of which could be shipped profitably.
Table 56 is reproduced from Information Circular 6926 to illustrate the charges made by smelters for excess deleterious elements.
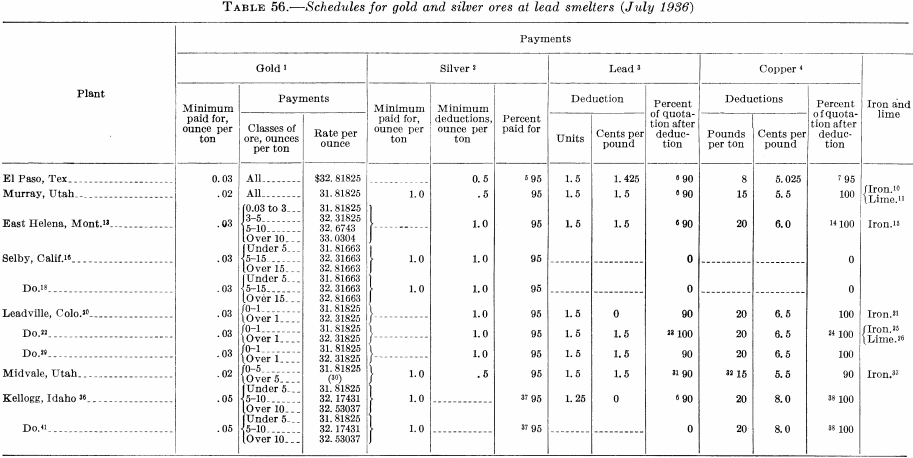
Gold and silver associated with base-metal ores usually are recovered in the smelter, where the base metal acts as a collector; later they are separated from the base metals in the refining process. Straight gold and silver ores or those containing only very small percentages of base metals (commonly known as dry siliceous ores) usually can be treated most economically at the mine by processes that result in the direct production of bullion. This avoids the cost of shipping ore and concentrates and payment of smelter charges and deductions, so that the net return to the miner on the gold and silver may range from a few to 15 or more percent higher than the amount that would be received were these metals recovered at the smelter. Sometimes it is profitable to recover part of the gold and silver by amalgamation or cyanidation and also produce a high-grade concentrate containing most of the associated sulfide minerals and the balance of the economically recoverable precious metal. The most profitable procedure will be determined in a given instance by local conditions, among the determining factors being the concentration ratio, the cost of milling and the percentage of recovery that can be made at the mine, the freighting cost, and the smelting charges, including deductions on ores and concentrates. These and other factors will be discussed briefly but in greater detail under Gold Ores.
The minimum grade of ore that can be smelted directly without previous ore-dressing treatment varies, depending largely on the natural fluxing characteristics of the ore, total tonnage of ore, distance to the smelter, haulage and freighting costs, and variations in treatment charges as between different ores and concentrates. An ore that is self-fluxing may be of lower grade for direct smelting than one that requires the addition of a large amount of barren flux. On the other hand, a low-grade ore sometimes may be profitably mined and shipped direct because it is high in silica, iron, or lime, or some other fluxing material required by the smelter. The total tonnage of ore available may be a governing factor, since if it is large enough and fuel and flux likewise are available within a reasonable distance, it may pay to erect a smelter near the mines, whereas if the tonnage is small the returns would not be large enough to amortize the capital outlay for a smelter and pay a profit on the venture. Isolation of the property and consequent high costs for freight obviously raise the cut-off grade between direct smelting and milling ores. Another factor is the concentration-ratio (tons of crude ore per ton of concentrate produced therefrom) and the percentage of recovery by concentration. The higher the concentration ratio without appreciably reducing the recovery, the greater is the advantage in concentration before shipment.
Selection of the best method to employ in a given instance requires not only highly specialized technical knowledge but also a knowledge of relative costs and ability to appraise correctly the various economic factors involved. The economic factors include average price of metals to be recovered, total tonnage to be treated, capital available, water supply, commercial affiliations of the company, royalties on processes and equipment, situation with respect to market and supply bases, transportation facilities, availability of power and fuel, facilities for disposal of tailing, character of labor supply, construction costs, and government regulations.
Ore-dressing methods fall naturally into two general subdivisions— (1) concentration methods and (2) direct-recovery methods (amalgamation and leaching or wet methods).
Concentration methods are employed to remove worthless gangue material and concentrate the valuable minerals of the crude ore in a smaller bulk. Thus, cobbing and hand sorting are crude yet in some instances very efficient concentrating processes. Drying to remove excess moisture in the ore is another simple form of concentration that reduces the relative weight of a worthless constituent of the ore (water) to that of the ore minerals.
Where all or most of the metal is present in the fines resulting from crushing, concentration may be effected by simple coarse crushing and screening, in which case the screen oversize is discarded.
However, the principal concentration methods and those having the widest application to all kinds of ores are gravity and flotation methods. Gravity concentration usually is effected in water or, less often, in a heavy-liquid medium and is based on differences in specific gravity of the ore and the gangue minerals. The formation of placer deposits is an example of natural gravity concentration, and the recovery of placer gold by panning, rocking, and sluicing exemplifies simple forms of artificial gravity concentration. Dry gravity concentration is exemplified by the separation of placer gold and associated heavy minerals from the lighter sands by agitation of the material by means of air pulsations through a riffled, porous deck.
Flotation concentration is effected by causing the selective attachment of a froth to certain minerals in a finely ground ore and agitated with water and frothing reagents. The froth, by its buoyant effect, causes the attached mineral particles to float, whereas the associated gangue minerals comprising the ore will sink. Another important method is magnetic concentration whereby particles of magnetic minerals are removed from a moving ore stream by electromagnets. Electrostatic separation also is employed sometimes, principally in dressing nonmetallic minerals.
Although the best general method of treatment for an ore may be apparent at the outset, it often is not and must be determined and details worked out by preliminary testing in the laboratory, in a pilot plant, or both, if a high extraction and low cost are to be obtained. One can seldom, if ever, visit a commercial ore-dressing plant, no matter how long it has been in operation, in which some changes are not being made or contemplated, unless the source of ore supply is nearly exhausted, which emphasizes the point that careful ore testing should always precede the design of a new plant. Seldom are there two districts in which the ores respond equally well to exactly the same details of treatment, and often the ores from mines in the same district or even those from different sections of the same mine require different treatment to obtain optimum results.
Gold Ore Processing
The fact that a product of high value and small bulk can be produced at a gold mine, often by relatively simple milling processes, has made it possible to operate gold mines successfully in remote and inhospitable regions where high costs of transportation, fuel, and power would make it impossible to exploit profitably base-metal ores of equal dollar value. As a result, in many instances gold-mine development has been the advance guard of civilization in regions where other mineral resources were developed later and agriculture and industry followed.
Types of Gold Ores
From the milling viewpoint, gold ores may be grouped as follows:
- High-grade ores suitable for shipment to smelters or for treatment by direct refinery methods without intermediate concentration.
- Free-milling ores in which most of the gold may be amalgamated with mercury after suitable grinding, but without roasting, leaching, or other preliminary treatment.
- Nonamalgamating ores, which, because of intimate association of the gold with pyrite, antimony, or arsenic, combination with tellurium, rusty oxide coatings, or the presence of graphite or greasy substances, must be subjected to treatment by other methods than amalgamation.
- Ores that will yield part of their gold content by amalgamation but have to undergo additional treatment by other methods to give a satisfactory over-all recovery. Most ores belong to this class, although the distinction between such ores and those of the preceding and following groups is based more often on economic rather than physical considerations.
- Ores that contain small amounts of amalgamable gold, with most of the gold in other forms, carrying, in addition, secondary amounts of base metals that are worth the expense of recovery.
- Highly siliceous ores, often of low grade, which are salable under certain conditions to lead or copper smelters for use as flux. They may command a premium for their fluxing value over and above the value of their contained gold.
- Base-metal ores in which the gold has minor or secondary value. These contribute heavily to total production, but since they are milled in plants designed primarily to recover other metals they are not considered further here.
- Placer gold. Recovery of metal from this type of material has been touched upon in an earlier section. A combination of gravity concentration and amalgamation generally is resorted to for treating placer material, the gold having been partially concentrated by natural agencies.
Methods of Ore Treatment
In the milling of gold ores, as with other kinds of ores, the treatment that will give the best metallurgical and economic results in any particular instance is determined primarily by the character of the ore, although other factors previously mentioned must be considered also.
The principal ore characteristics that govern the selection of the milling method are grade of ore and uniformity of tenor, the size of the gold particles, the nature of the associated minerals, and the degree to which the gold particles are locked within them. The details of the process also will be determined by these characteristics and to a considerable extent by the crushing and grinding qualities of the ore.
Ores of very low grade usually can be exploited successfully only when treated on a large scale in plants of high capacity, which involve heavy initial capital outlay. Higher-grade ores may be treated profitably in smaller plants with lower initial outlay per dollar of output, unless the ore is refractory and a complex, expensive treatment is un-avoidable. Extremely rich ore mined from “pockets” usually is hand-sorted and treated in the mill refinery or is shipped direct to a smelter.
In mills treating rich gold ores it is usually necessary to employ more than one process to recover a high percentage of the gold. The tailing from the first or primary process employed to treat such ores usually carries enough gold to warrant secondary treatment by another process with or without regrinding or by the same process after re-grinding. With low-grade ores it may be necessary to employ two or more processes also, the first to produce a product from which a large part of the gangue has been removed and the following ones for extraction of the gold or production of a high-grade concentrate.
Plants for treating ore of uniform grade are the simplest to design and operate, other things being equal. Sudden additions of considerable high-grade ore to an average medium or low-grade mill feed tend to throw the process out of balance and usually will cause increased tailing loss. However, if the high-grade ore is free milling, the feed to the next process may be kept more uniform by first removing much of the gold by amalgamation; or, if the high grade contains coarse gold, by catching it in traps before the ore passes to the main process.
The size of the gold particles in the ore often has considerable influence on the method of treatment to be employed. Because gold is malleable, ordinary grinding will not reduce coarse gold particles to a size that can be dissolved completely by cyanide in the time usually given for solution of fine gold in cyaniding practice, and it is, therefore, desirable to remove coarse gold first by means of traps, blanket tables, jigs, or other devices.
If the gold occurs in extremely small particles, the ore must be ground fine to liberate them from the gangue and expose their surfaces to contact with cyanide solution, or with mercury, as the case may be, or to permit making a high-grade concentrate without carrying into the tailing prohibitive amounts of gold attached to the gangue particles. The fineness of grinding may be determined to some extent by the milling method, which in turn is influenced by the associated minerals, their grindability, and their effect on the applicability of the cyanide, amalgamation, or concentration processes. If the gold is closely associated with sulfides of the base metals, relatively coarse grinding may suffice for gravity or flotation concentration; whereas, if associated with quartz or silicate minerals, much finer grinding may be required to liberate the gold for amalgamation or cyanidation. If the gold is in such fine state and so intimately associated with the gangue minerals, whether sulfides or other minerals, that sliming is necessary to liberate it, cyanidation usually is the best method of recovery to employ unless the ore contains deleterious constituents in quantities sufficient to inhibit solution of the gold, foul the solutions, or react with the cyanide. In some ores of this kind, however, the finely divided gold is contained principally in sulfides that can be concentrated at relatively coarse sizes by flotation, permitting the discard of a large part of the gangue before finer final grinding and treatment of the concentrate by cyanidation.
The nature of the associated ore minerals is one of the most important factors in the selection of treatment methods. Certain constituents of ores may, by causing poor extraction or high operating costs, rule out of consideration a treatment that otherwise would be suitable, or may compel the use of expensive steps that otherwise would not be required.
Pyrite is present in most unoxidized gold ores, where it nearly always contains some gold. Complete liberation of this gold from the pyrite usually entails fine grinding, the cost of which may be prohibitive; and, even if freed, the gold particles often retain a surface film of sulfide capable of preventing amalgamation. Generally it has been found that ores containing much vein pyrite will not yield a satisfactory gold recovery by amalgamation alone but must be treated further by concentration or cyanidation. Where flotation is employed, it may be followed by cyanidation of the concentrates, of the tailing, or both, or by smelting of the concentrates.
Other base-metal sulfides have effects similar to those of pyrite and, in addition, may have a slight tendency to foul mercury, but usually only the sulfides of arsenic, antimony, and bismuth are likely to cause much trouble. If the sulfides are partly oxidized, however, enough acids and soluble sulfates may be formed to give serious trouble in the cyanide process. Pyrrhotite (FenSn+1) is slowly dissolved by cyanide solutions, consuming cyanide, and also has the power of absorbing the oxygen that enters into the reaction.
Base-metal sulfides are seldom abundant enough in gold ores to make it profitable to save and market them separately. Occasionally, however, their presence, even in small amounts, has caused the treatment to be modified slightly where they can be recovered cheaply to furnish an additional source of revenue. At the Alaska-Juneau mine lead is present in very small quantities (a fraction of 1 percent) but the tonnage treated is large (currently about 11,000 tons per day) and the aggregate amount of lead is worth saving in the form of a concentrate, which is shipped for smelting.
Arsenic and antimony if present in appreciable quantities cause serious trouble. Sulfarsenides and sulfantimonides are present in many gold ores, and these, together with the sulfides, cause “sickening” of the mercury in the amalgamation process, chiefly by coating it with a black film that causes the mercury to separate into small globules. Partly decomposed arsenic and antimony minerals and the oxides realgar and orpiment are particularly injurious. Some arsenic and antimony compounds are soluble in cyanide solutions and cause high consumption of cyanide; they also tend to foul the solutions to such an extent that gold and silver cannot be dissolved, or they may form reducing compounds that will precipitate gold already dissolved.
Tellurides of gold and silver, important ore minerals in some districts, do not amalgamate directly and cause sickening of the mercury as well. Sodium amalgam will amalgamate with them, however. It long has been thought that they are not amenable to ordinary cyanidation but are soluble in bromocyanide, and the usual practice has been to roast telluride ores before cyanidation. Recent research has demonstrated that tellurides are soluble in cyanide if ground fine enough, but it is probable that the extra expense of fine-grinding often will exceed the cost of preliminary roasting to obtain equally good extraction.
Certain metallic oxides, carbonates, hydrates, sulfates, and arsenates, as well as soluble sulfides, form a group known collectively as cyanicides. They react with cyanide, forming double cyanides, which dissolve gold and silver very slowly, if at all, or they may decompose the cyanide and thus destroy its value as a solvent. Soluble copper salts are perhaps the most troublesome cyanicides, for, in addition to reacting with the cyanide, they deoxidize the solutions. Along with ferrous sulfide, sulfate, and other salts, their effect cannot be counteracted by the addition of lime to the pulp but they can be partly removed by a preliminary acid or ammonia wash. Partial oxidation of sulfide minerals may prevent successful direct concentration by flotation, although special conditioning reagents may be used to make them amenable to this process.
The principal gold-milling methods include hand-sorting, amalgamation, gravity concentration, flotation concentration, cyanidation, and various combinations of two or more of these methods; chlorination is seldom employed in modern plants, though at one time it was used to a considerable extent.
In all of these methods except hand sorting, crushing and grinding of the crude ore are the preliminary steps, and in some instances where coarse crushing is done in stages, sorting may follow the first stage.
Amalgamation
There are two principal methods of amalgamation—plate amalgamation and barrel amalgamation.
In plate amalgamation the ore is crushed wet in stamp mills or ground in ball mills and the resulting pulp flows over copper plates (often silvered), which are coated with amalgam and mercury. Particles of free gold and silver coming in contact with the mercury alloy with it to form gold-silver amalgam, which adheres to the plates. Where stamps are employed, the screen openings range from about 12 to 40 mesh as a rule, which determines the maximum size of ore particles in the pulp passing over the plates.
Mercury sometimes is introduced into the stamp mortar, also, especially if there is much coarse free gold in the ore. The plates are dressed every few hours, the interval depending on the grade of the ore, amount of sickening that takes place, the scouring action of the pulp, and degree of discoloration of the plates by chemical action. Dressing usually comprises brushing the plates from the bottom up-ward with a whiskbroom or rubber scraper to remove excess amalgam, sprinkling fresh mercury on the resulting bare or thin spots, and working the mercury until the surface shows the proper amalgam condition. At longer intervals the plates are thoroughly cleaned and re-dressed with mercury. The gold is finally recovered by squeezing out excess mercury, retorting the amalgam, melting down the resulting sponge gold and casting it into bars.
The stamp mill is not an efficient crushing machine, although it has been retained in a number of mills, some of them quite large, probably owing to the fact that the investment had been made or to the peculiar crushing characteristics of the ores. It has some advantages in mills of small capacity because of the small unit capacity of each battery, which permits shutting down one or more batteries without lessening the efficiency of the others, and because of the comparatively low first cost.
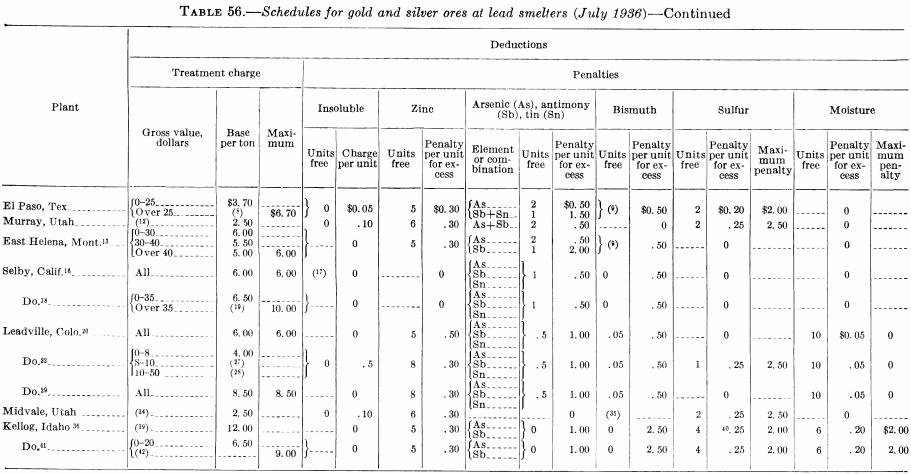
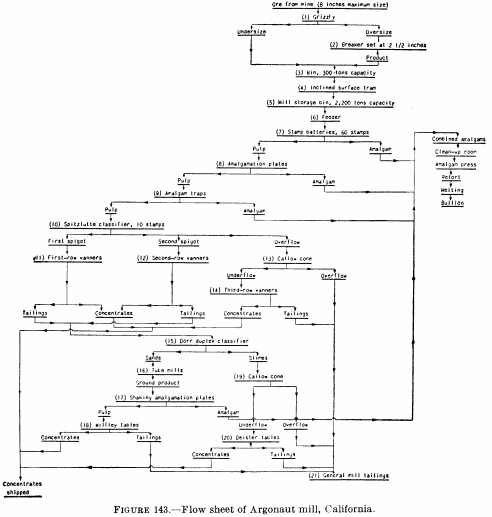
Figure 143 is the flow sheet of the Argonaut mill as it was in 1928, which was typical of stamp-mill plate-amalgamation practice in the Mother Lode (California) district at that time. After passing over the plates, the pulp went to vanners for gravity concentration, and the vanner tails were cyanided in a separate plant. Figure 144 is the flow sheet of the south mill, Homestake mine, in 1927, which employed two-stage grinding and amalgamation; crushing is done by stamps and grinding by rod, tube, and ball mills.
Barrel amalgamation usually is employed for treatment of heavy concentrates from corduroy or sand tables or from coarse-gold traps and other small batches of rich material. The barrel is a revolving steel cylinder. It is charged with a batch of concentrate and a light load of grinding balls to break up the material and to scour and brighten the surfaces of the gold particles. After the barrel has rotated for several hours, an excess of mercury is added to it and it is revolved for 2 or 3 hours more. The batch is then discharged, the mercury and amalgam are recovered by panning, and the gold is
recovered from the amalgam by retorting. Pan amalgamation is employed similarly for treating small batches of very rich concentrate; the concentrate is ground with mercury in cast-iron tubs or pans equipped with rotating dies or grinding shoes.
Concentration
Concentration is employed for the purpose of segregating the gold in a product of relatively small bulk for direct shipment to a smelter or for further treatment in the mill. For many years gravity concentration was virtually the only concentrating method employed in gold mills, but in recent years flotation methods have been used widely, especially in the flow sheets of new mills.
As an adjunct to other methods, concentration may effect marked savings in operating costs. Thus, it is often possible to produce a concentrate containing most of the gold after relatively coarse grinding, then fine-grind the concentrate preparatory to extracting the gold by cyanidation or amalgamation. Since fine-grinding usually is the most costly operation in gold milling, it is obvious that where it can be restricted to a concentrate, the bulk of which is only a fraction (sometimes a very small fraction) of that of the original ore, the saving in cost is appreciable. Furthermore, the constituents of the ore that are
most abrasive and difficult to grind are often the gangue minerals that are largely removed by concentration.
The principal gravity-concentrating devices now employed are stationary blanket tables, concentrating cones, jigs, shaking tables, and vanners. Concentrating cones or traps frequently are employed to remove coarse gold from the circuit before it passes to the main treatment process; they are commonly placed between the primary ball or rod mill and a classifier operating in closed circuit therewith. Jigs are installed for a similar purpose at some point near the head of the flow sheet.
Blanket tables are stationary sloping surfaces covered with corduroy, coco matting, or other coarse-woven fabric. Corduroy especially manufactured for this purpose is probably the best material to use because of its durability and the riffling action of the ribs of the cloth. The pulp from the grinding mills is passed in a thin stream over the table, and the coarse gold and heavier minerals are caught and held on the cloth. The blankets are taken from the tables and washed in a tub or vat at frequent intervals to remove the concentrate, which is then treated in an amalgam barrel, cyanided, or shipped to a smelter.
Shaking tables are of several types, but all operate on the same basic principle. They consist of a flat, sloping deck, fitted with thin, tapered riffles running parallel or diagonally with the long dimension of the deck (for sand feed) or are covered with fabric (for slime feed). An eccentric mechanism is employed to oscillate the deck in a longitudinal direction. The pulp, which should be sized or classified for best results, is fed in a thin stream over the deck, the light gangue minerals being washed over the lower side of the table whereas the gold and heavy minerals are caught behind the riffles and moved along toward the end of the table by the jerking motion.
Figure 145 is a flow sheet of the Alaska-Juneau mill (1929), in which tables are employed to produce a high-grade gold concentrate that is treated by amalgamation, a middling or shipping concentrate, and a tailing product.
Vanners formerly were employed extensively for concentration of slime material, but a low extraction usually resulted because of the tendency of very fine gold particles to float off with the gangue. Usually, appreciably higher extraction of gold from slimes can be obtained by flotation or cyanidation.
Classification is a form of concentration that usually is employed more specifically for the purpose of separating the coarse from the fine material (the sands from the slimes) and in the usual gold-mill flow sheet is employed in the grinding section to separate coarse material that requires regrinding from the fine slimes that are ready for the next stage of the process. Classifiers may be either in closed circuit with a grinding mill (usually a ball, pebble, or rod mill), in which case the sands are returned to the mill from which they were received, or in series with a second mill.
Flotation is used principally on gold ores that carry enough sulfide mineral to stabilize a froth, although it has been applied successfully in connection with other methods to the treatment of oxidized ores containing little if any sulfide mineral. The flotation concentrate may be a finished product that is shipped to a smelter or it may be treated further for extraction of the gold by direct cyanidation or by roasting followed by cyanidation. Flotation reagents are now available that will not inhibit amalgamation, and flotation concentrates are sometimes treated by amalgamation for recovery of the gold.
In some mills a quick recovery of coarse, rich material is accomplished in a “unit cell” placed in the grinding section, usually between the ball mill and classifier or after the classifier and just ahead of the main flotation circuit, which thus performs a function similar to that of a concentrating cone or jig placed at the same point in the circuit as mentioned previously.
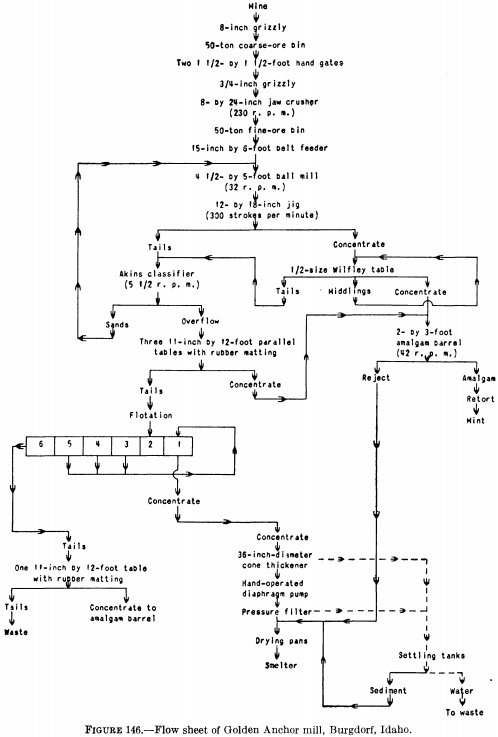
Figure 146 is the flow sheet of a small mill at Burgdorf, Idaho, in which a jig follows the ball mill and the concentrate from the jig is cleaned on a Wilfley table and amalgamated in a barrel. The jig tailing and the Wilfley table tailing are classified, the sands being returned to the ball mill for regrinding while the slime (“overflow” in figure) is concentrated on tables covered with rubber matting. The tailing from the tables is treated in a five-cell flotation machine. In this mill, therefore, flotation is employed as an adjunct to gravity concentration and amalgamation to recover additional gold from the tailing.
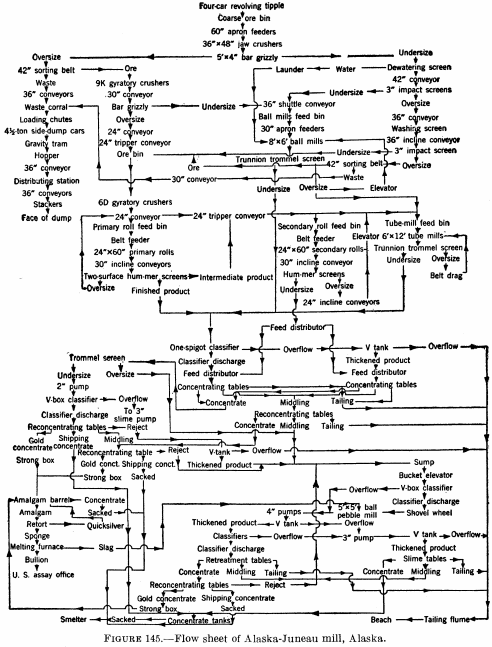
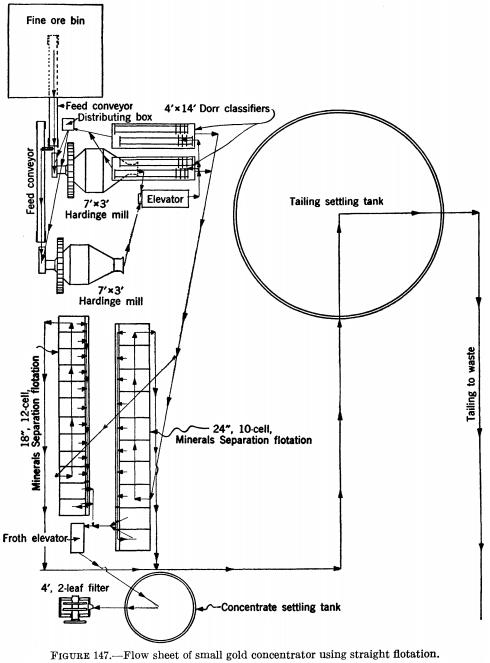
Figure 147 is the flow sheet of a small gold concentrator in which straight flotation is employed, the overflow from the classifiers being fed directly to two sets of flotation cells in parallel, the cleaner cells of which produce a finished concentrate for shipment to the smelter.
There are so many different ways in which flotation is combined with other methods of recovery that neither figure 146 nor figure 147 can be said to be typical; they merely represent two extremes, one in which flotation is an adjunct to the mill for recovering gold in the mill other in which flotation is the only process. Flotation concentrates often are treated by cyanidation, usually after regrinding, in which case reagent strength and consumption per ton usually are higher than when raw ores are cyanided.
Cyanidation
The cyanide process is based on the work of MacArthur and the Forrests (begun in 1886) and involves the leaching of gold and silver from alkaline pulps with dilute solutions of sodium or potassium cyanide, the filtering and clarification of the gold-silver-bearing solution, and precipitation of metals from solution. Oxygen is essential to the solution reaction, and in practice oxygenation is accomplished by violent agitation of the pulp by the injection of compressed air. Precipitation usually is by means of zinc shavings or zinc dust and is improved if the solution be first de-aerated. Aluminum is used instead of zinc under some unusual conditions.
Nearly all gold ores are amenable to cyanidation, although, as previously pointed out, certain constituents of some ores cause excessive consumption of cyanide and other reagents, or the fouling of solutions, with resultant high costs. A few are so difficult to treat by direct cyanidation that costly preliminary treatment must be employed, when other methods might prove more economical. The principal cyanicides have been mentioned already. Carbonaceous matter, often contained in graphitic slates associated with gold ores, may result in high tailing loss through its capacity to precipitate gold from the solutions.
In general, cyanide treatment comprises four steps—preparation of the ore (crushing, screening, grinding, and classification), leaching or dissolving the gold, separation of the gold-bearing (“pregnant”) solution from the leached pulp (thickening, filtration, and clarification of the solution), and precipitation.
Preparation of the ore as above defined is very important, and as it is usually the most expensive part of the process (especially fine grinding), it has been the object of much research and experimentation. Gow, Guggenheim, and Coghill reviewed the subject of fine grinding in ore concentrators in 1933.
Data on equipment, fineness of grinding, costs, etc., at gold mills will be tabulated with other gold-milling data later.
Where an all-cyanide method is employed, the grinding usually is done in cyanide solution and no water is used in the mill except for final washing of the filter cake. A good deal of the gold, sometimes most of it, is then dissolved in the grinding section of the mill and the time of contact of solution with pulp in agitators and thickeners may be reduced appreciably.
Leaching is accomplished in different ways, according to the character of the ore; the entire mill feed may be finely ground and treated by the agitation or all-slime process, it may be separated by classification into sand and slime portions that are cyanided separately or concentrate may be cyanided by one method and tailing by another. There are two principal leaching methods—batch or “sand” leaching and all-slime or agitation leaching.
Sand leaching is done in tanks fitted with fiber bottoms, into which the sands are charged. Successive leachings with cyanide solution are made, each usually being weaker than the preceding one. The solutions are either introduced at the top and percolate down through the pulp, or enter at the bottom under a slight pressure and are decanted after passing upward through the pulp. Draining and leaching in stages usually gives better results than continuous passage of the solution, since the charge is better aerated in this way. For successful leaching, the charge should be of classified material and free from slimes that would clog the pulp column, preventing passage of the solution in places and causing “channeling” in others. Thus, in large plants where sand leaching is practiced, it is customary to treat only the sands by this process, the slimes being treated by agitation and countercurrent decantation or (and) filter washing. The sand charge is given one or more final water washes and is discharged from the tank.
All-slime or agitation leaching depends on constant movement of the ore particles in well-aerated cyanide solution. Agitation and solution usually begin in the grinding circuit, as previously stated. After grinding and classification of the pulp to the desired degree of fineness, it is usually first thickened and then fed to agitation tanks, where it is brought to the required consistency and cyanide strength by addition of stock cyanide solution. Agitation is accomplished by mechanical means, by compressed air, or both. The time of agitation required to obtain extraction of the gold varies and must be determined in each instance by tests and experience. Agitation in batches, while it can be controlled rather closely, is usually more costly than the continuous process that is used almost universally in modern plants.
In the agitation processes the pulp from the primary thickener goes to agitation tanks, of which there are usually at least three in series. After leaving the last agitator, the pulp may be thickened and washed one or more times and finally filtered, or it may be subjected to continuous countercurrent decantation. Most of the cyanide plants in Ontario and Quebec employ filter washing rather than countercurrent decantation. If grinding has been done in cyanide solution, the overflow from the primary thickener preceding the agitators usu-
ally constitutes the gold solution that goes to the clarification and precipitation section of the mill.
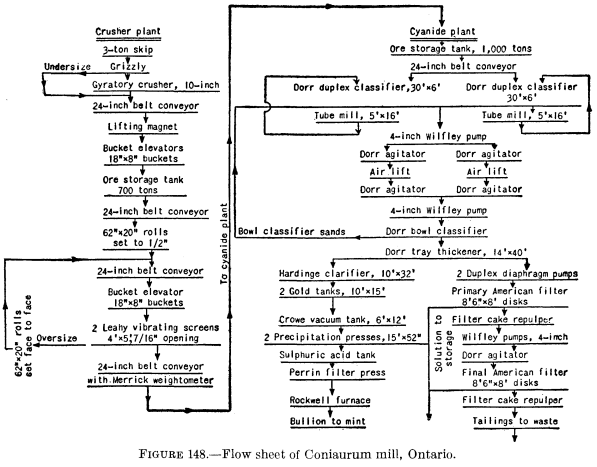
In straight filtration-leaching, the process is briefly and essentially as follows: The pulp from the agitators may be thickened first or may be sent directly to the continuous filters. On the filters the cake first receives one or more washes with barren cyanide solution and then one or more of water, both filtrates being pumped back to the mill-solution tank for use in the grinding and other sections of the mill. Figure 148 is a flow sheet of the Coniaurum mill (1931) in which filtering, repulping and agitation, and secondary filtering are employed.
In countercurrent decantation the pulp from the agitators goes to three or more thickeners arranged in series. These thickeners are often of the classifier-thickener type. The leached pulp enters the first thickener from the last agitator (if there is no primary thickener preceding the agitators, this may be considered the primary thickener). Most of the pregnant solution overflows this thickener and either goes back to the grinding circuit or directly to clarification and precipitation, while the thickened underflow is discharged to the second thickener of the series. In this thickener the pulp is mixed with much lower grade overflow from the third tank. After mixing in the second tank, most of the dissolved gold that came in with the pulp is dispersed throughout the solution, raising its value. The overflow from the second tank goes, in turn, to the first tank, while the thickened pulp is discharged to the third tank of the series, carrying with it some solution of lower grade than that which it brought with it from the first thickener. The process is repeated in each tank, the pulp becoming successively leaner as regards the value of the solution carried along with it whereas the main body of solution is enriched at each step. At the last thickener in the series enough fresh water or weak barren solution is added to offset the amount of liquid leaving the circuit with the leached slime plus spillage, evaporation, and other losses, and the slime goes to the tailing pond or to filtration for further washing. Countercurrent decantation is illustrated by the simple flow sheet of the Big Jim mill (fig. 149).
The subject of gold milling was discussed in a general way but in somewhat more detail in an earlier bulletin. Progress reports covering results of research on gold milling are issued from time to time by the Metallurgical Division of the Bureau of Mines.
Gold-Milling Data
The data in table 57 have been obtained from various sources as noted. In view of the frequent changes in practice that take place in most mills due to changes in ore characteristics, in methods and equipment brought about by research and improved machines, and increases or decreases in capacity, these data should be understood to apply specifically to the period covered and only generally to long-time operations. However, they represent broadly current practices and results obtained in milling various types of gold ores.
