
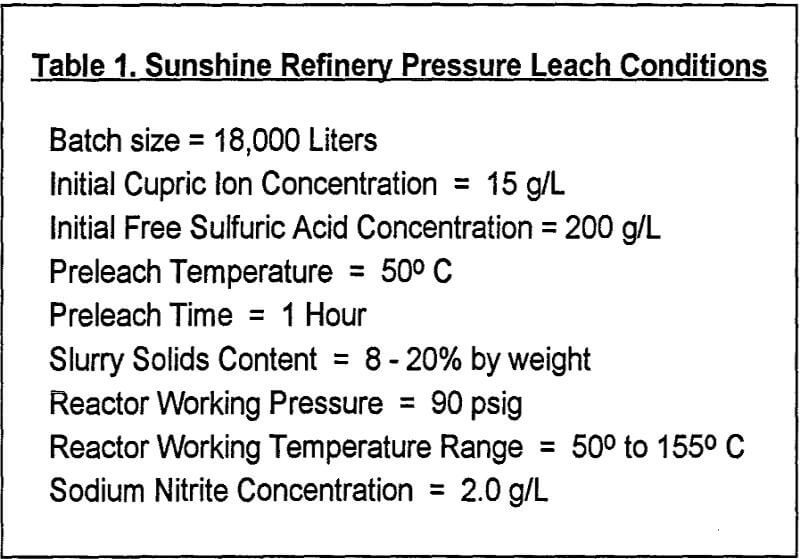
Simplistically, nitrogen species oxidize the sulfide and solubilize some of the metal values. In essence, the silver, copper and less noble metals are dissolved in the leach. The slurry is screened, thickened and filtered. Two different solids are produced. First, sulfur pellets are recovered. Then, the insoluble fine leach residue, consisting primarily of lead sulfate, is filtered away and shipped to a smelter. The solution is selectively and efficiently stripped of silver using sodium chloride (NaCl) solution to precipitate silver chloride (AgCl). This operation is implemented through a novel ion selective electrode developed at Sunshine. The solid AgCl precipitate is filtered from the pregnant solution and washed thoroughly with water. Conversion to elemental silver is facilitated by a novel aqueous reduction process utilizing soda ash and dextrose. The silver is further electrorefined to a purity of 99.95%, 99.99% or 99.999%.
The silver free leach solution is treated with slaked lime to reduce much of the acidity. Copper is then recovered from solution using conventional solvent extraction and electrowinning technology. A high grade copper metal cathode is produced. The waste solutions from the entire process are treated with slaked lime pH adjustment, precipitation and settling to produce an environmentally acceptable waste water discharge.
Electric Furnace Matte Leaching
With the success of leaching the TBRC matte, the next phase of testing involved leaching of the upstream Electric Furnace matte. This material contains much more iron than TBRC matte. Subsequent testing showed that the Sunshine process could handle this high iron material.
In Sunshine leaching of the Electric Furnace matte, again, two phases were produced from leaching. The liquid phase contained the base metals. The solid phase contained the PGM’s. No elemental sulfur pellets were produced. However, more severe conditions were used than with the TBRC matte. This resulted in greater base metal leaching and also led to low amounts of the rhodium and palladium initially reporting to solution instead of to the solid phase. This necessitated recovery of solubilized PGMs before downstream processing for the copper, nickel and iron. The solids from this recovery step were handled by blending back to the leach step or blending with the leached solids. On a plant scale, either method would suffice depending on the ruling material balance at hand.
For testing of the Electric Furnace matte, a pilot scenario was performed whereby both recycle and blending of recovered rhodium and palladium was performed. Both solids and liquid assays were performed by Stillwater Mining Company. Results were excellent and are presented in Tables 5, 6, and 7.
As was seen in the TBRC testing, the base metals are removed from the Electric Furnace material. Again, the result is a high grade PGM residue which can be easily refined. The solution from Electric Furnace matte leaching was readily treated using the same techniques employed for the TBRC leach solution. Once more, proven hydrometallurgical technology can be used to recover copper and nickel as valuable products and leave iron as a waste product. This indicates that Electric Furnace matte processing could easily be integrated into the Sunshine Refinery infrastructure. The matte converting step at the Stillwater smelter could be eliminated, and some associated processing could be reduced (i.e. granulating, drying, PGM slag recycling, SO2 scrubbing, gypsum production etc.). This would increase throughput, lower operating costs and allow domestic refining of the resultant PGM concentrate.
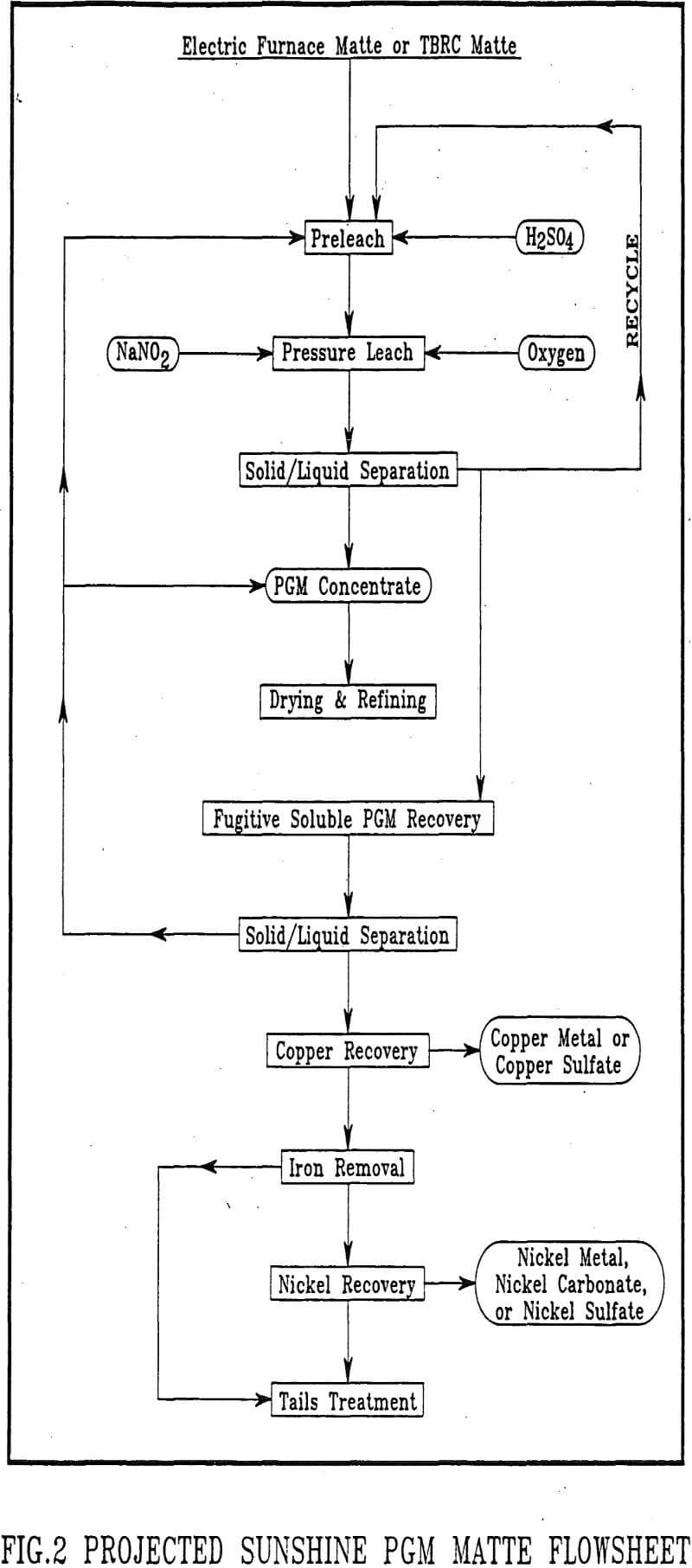
Testing of Stillwater smelter PGM mattes using Sunshine nitrous-sulfuric acid pressure leaching was successful. A proposed flowsheet is presented in Figure 2. High-grade PGM solids, which could be refined domestically, are produced from both the TBRC and Electric Furnace mattes. Scale-up of this patented leach process to operational size could readily be accomplished. In addition, iron, copper and nickel would be handled by proven hydrometallurgical technology. In the case of nickel and copper, marketable products are obtained.

