The electrolytic separation of nickel and cobalt by electrorefining in a molten salt electrolyte was shown to be technically feasible. Nickel-cobalt alloys containing up to 5.5 percent cobalt were electrorefined in a KCl-LiCl-NiCl2 electrolyte to a high-purity nickel metal meeting atomic energy specifications. The amount of cobalt transferred to the cathode deposit was found to be dependent on the nickel content of the electrolyte, the cobalt content of the electrolyte, the ratio of nickel to cobalt in the electrolyte, and the cathode current densities.
By controlling the nickel-cobalt content of the electrolyte, nickel metal containing less than 0.10 percent cobalt was routinely prepared from nickel-cobalt alloys containing up to 5.5 percent cobalt. In the process, the nickel content of the electrolyte was gradually replaced by the solution of cobalt from the anode.
The cobalt content of the electrolyte could be recovered by first refining with a cobalt anode to remove nickel from the electrolyte, and then electrowinning the cobalt from the electrolyte. The preparation of tungsten-nickel or tungsten-cobalt alloys was accomplished by using a tungsten anode and electrowinning in KCl-LiCl-NiCl2 or KCl-LiCl-CoCl2 electrolyte.
The use of a normal atmosphere cell or a protected atmosphere cell was found to be feasible for the electrorefining process. The major difference in the performance of the two types of cells was in a slightly greater loss of some of the normal cell electrolyte rs nickel content by oxidation or sublimation.
Electrolytic methods using molten salt electrolytes were investigated for the separation of nickel and cobalt in nickel alloys containing up to 5 percent cobalt. The objective was to prepare nickel containing less than 1 percent and preferably below 0.20 percent cobalt. Nickel containing less than 1 percent cobalt is suitable for most metallurgical applications and metal containing less than 0.20 percent meets atomic energy application specifications.
Nickel and cobalt occur together in laterite and serpentine ores and are generally extracted together because of their physical and chemical similarities. Mahan converted nickel oxide from laterite and serpentine ores to nickel metal by first converting the oxide to sponge nickel and then melting the sponge to remove gangue and part of the cobalt. He was able to produce a nickel metal containing about 1 percent cobalt. Brooks and Rosenbaum have described a process for the separation and recovery of cobalt and nickel by solvent extraction and electrorefining using a nickel oxide starting material similar to that used by Mahan. Aqueous electrolyses to produce nickel containing below 0.20 percent cobalt has been dependent on chemical means for removing the bulk of the cobalt before electrolysis.
The lack of success in developing an electrolytic process for the direct separation of nickel and cobalt in aqueous solutions has been attributed to their nearly identical standard electrode potentials. The use of molten salt electrolysis to separate cobalt and nickel appeared feasible from reported standard potential values on the electromotive force series of metals in fused salts. The standard electrode potentials of nickel and cobalt in molten salt electrolytes differ sufficiently to offer the possibility of separating nickel and cobalt by electrolysis in such electrolytes. Laitinen and Liu determined the standard electrode potentials of the metals at 450° C using the eutectic mixture of LiCl-KCl as the solvent. They reported a value of minus 0.795 volt for Ni (II) – Ni (0) and a value of minus 0.991 volt for Co (II) – Co (0) using the Pt (II) – Pt (0) system as the reference electrode. A similar spread in the standard electrode potentials of the two metals in fused 1 to 1 KCl-NaCl was reported by Flengas and Ingraham who also determined the activity coefficients of the metal chlorides in that solvent at 700° C as 0.046 for CoCl2, and 0.34 for NiCl2. Both the difference in electrode potentials and activities of nickel and cobalt in molten solvents indicated the possibility of selectively electrodepositing nickel from nickel-cobalt materials. Atkinson, in a paper on the electrolytic transfer of platinum metals in fused chloride electrolyte, indicated that nickel nearly free of cobalt could be prepared from nickel containing 0.41 percent cobalt by using a fused chloride electrolyte. Techniques developed by the Bureau of Mines for molten salt electrorefining have been successful in the purification of metals such as beryllium, chromium, molybdenum, titanium, and vanadium.
Equipment
Two types of electrolytic cells were used in the investigation. One was a helium atmosphere cell designed to permit electrolysis in the molten salt under a protective atmosphere of helium. The other did not utilize any special means for the protection of the electrolyte or the refined metal product from air oxidation and will be referred to as the normal atmosphere cell.
The helium atmosphere cell was constructed so that the anode and the cathode could be inserted or removed from the cell without exposing either the electrolyte or the hot, refined metal product to air. The electrolyte was kept under a helium atmosphere at all times. A schematic drawing of the cell is shown in figure 1. The cell consisted of an electrolyte compartment, a slide valve, and a water-cooled lock. The electrolyte compartment consisted of a nickel crucible made from a 24-inch length of 6-inch-diameter, schedule 40 nickel pipe with a welded nickel bottom. A high-density graphite liner was
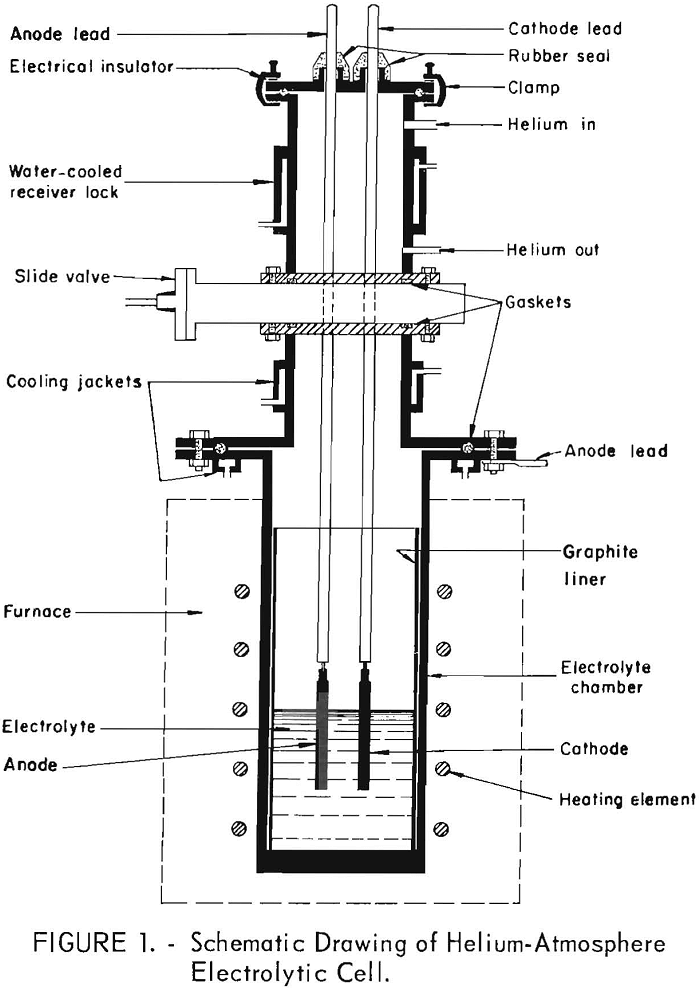
inserted in the crucible to contain the molten electrolyte. A water-cooled, mild-steel flange was welded to the top of the crucible for use in connecting it to the slide valve. The slide valve was inserted between the compartment and the lock and provided a gas and vacuum seal for isolating one from the other. The lock was water-jacketed for rapid cooling of metal deposits and had valved inlets for helium and vacuum connections. A clamp-on cover was used to close the lock. The cover was electrically insulated from the lock by a rubber gasket and insulators at the clamps. The cover had fittings for anode and cathode lead rods which were sealed with rubber sleeves that permitted raising or lowering of the electrodes without contamination of the helium atmosphere. There were two sight glasses in the cover for observation and manipulation of the deposits.
The normal atmosphere cell consisted of a flanged electrolyte compartment similar to that used in the helium atmosphere cell. A fused silica liner was used to contain the electrolyte. A cover plate with anode and cathode fittings was placed directly on top of the electrolyte compartment. No slide valve or lock were used.
Electrolyte
The molten electrolyte selected for use in this investigation was composed of NiCl2 added to the eutectic mixture of LiCl-KCl (44 weight-percent LiCl-56 weight-percent KCl). The initial nickel content of the electrolytes was between 4 and 6 weight-percent. The electrorefining was performed at 450° C. This electrolyte had several advantages over other molten electrolytes which had higher operating temperature, such as KCl-NaCl-NiCl2 (750° C) and NaCl-NiCl2 (850° C) . One advantage was the possibility of electrorefining without the need for providing a protective atmosphere over the molten electrolyte to prevent the loss of its NiCl2 content by air oxidation. Another reason for selecting the lower temperature electrolyte was to diminish the corrosiveness of molten electrolytes containing NiCl2. Requirements for materials of construction to contain the molten electrolyte and its vapors in the atmosphere over the electrolyte were less severe with an electrolyte operating at 450° C. Less power was also needed to maintain the molten electrolyte at the lower temperature.
Operational Procedure
The electrolyte for the helium atmosphere cell was prepared by melting a dried mixture of the component salts at 400° C under vacuum in the cell. Approximately 5,000 grams of electrolyte was used in each series of tests. When gas evolution from the molten electrolyte ceased, the cell was pressurized with helium, the temperature was raised to 450° C, and a helium atmosphere slightly above atmospheric was maintained over the electrolyte.
To prepare the cell for electrolysis, the slide valve was closed to maintain the helium atmosphere over the electrolyte, the cover was removed from the lock, and a nickel-cobalt alloy bar was attached to the anode electrode lead. A nickel cathode was attached to the cathode electrode lead. The cover was clamped on the lock, and the lock was evacuated and back-flushed three times with helium to remove the air. The slide valve was opened and the anode and cathode were positioned in the electrolyte. Electrolysis was started using full wave, rectified direct current supplied by a selenium rectifier. When electrolysis was terminated, both the anode and the cathode, with its metal deposit, were raised just out of the electrolyte and allowed to drain 10 minutes to remove excess electrolyte. They were then withdrawn into the lock and the slide valve closed. The anode and the nickel deposit were removed from the lock after cooling to 25° C. The anode bar was leached free of salt, dried, and weighed. The anode and a new cathode were attached to their respective leads and a new operating cycle started. Cathodes configurations used were various diameter nickel rods 6 inches long, and one was a nickel plate 3 inches wide by 6 inches long. One complete deposition cycle is referred to as a test and a group of tests using the same anode material and the same electrolyte is referred to as a series, in this report. Sampling of the electrolyte for analysis was done between tests.
The electrolyte was prepared in the normal cell simply by melting the dried salt mixture and raising the temperature to 450° C. Electrolysis in the normal cell was similar to that in the helium atmosphere cell. The major difference was that the electrolyte and the hot deposit of refined nickel were exposed to air when the deposit was removed at the conclusion of each test.
The deposits from both types of cells consisted of dendritic crystals of nickel attached to the cathode. When the metal deposits were withdrawn from the molten electrolyte they were coated with a thin film of salt. This coating protected the hot, dendritic metal crystals from oxidation when they were exposed to air on removal from the normal cell.
The metal was stripped from the cathode and leached in a diluted solution of hydrochloric acid (HCl) 1 to 50 to remove the electrolyte. It was then rinsed free of chlorides and dried.
Experimental Results
Electrolysis in a Helium Atmosphere Cell
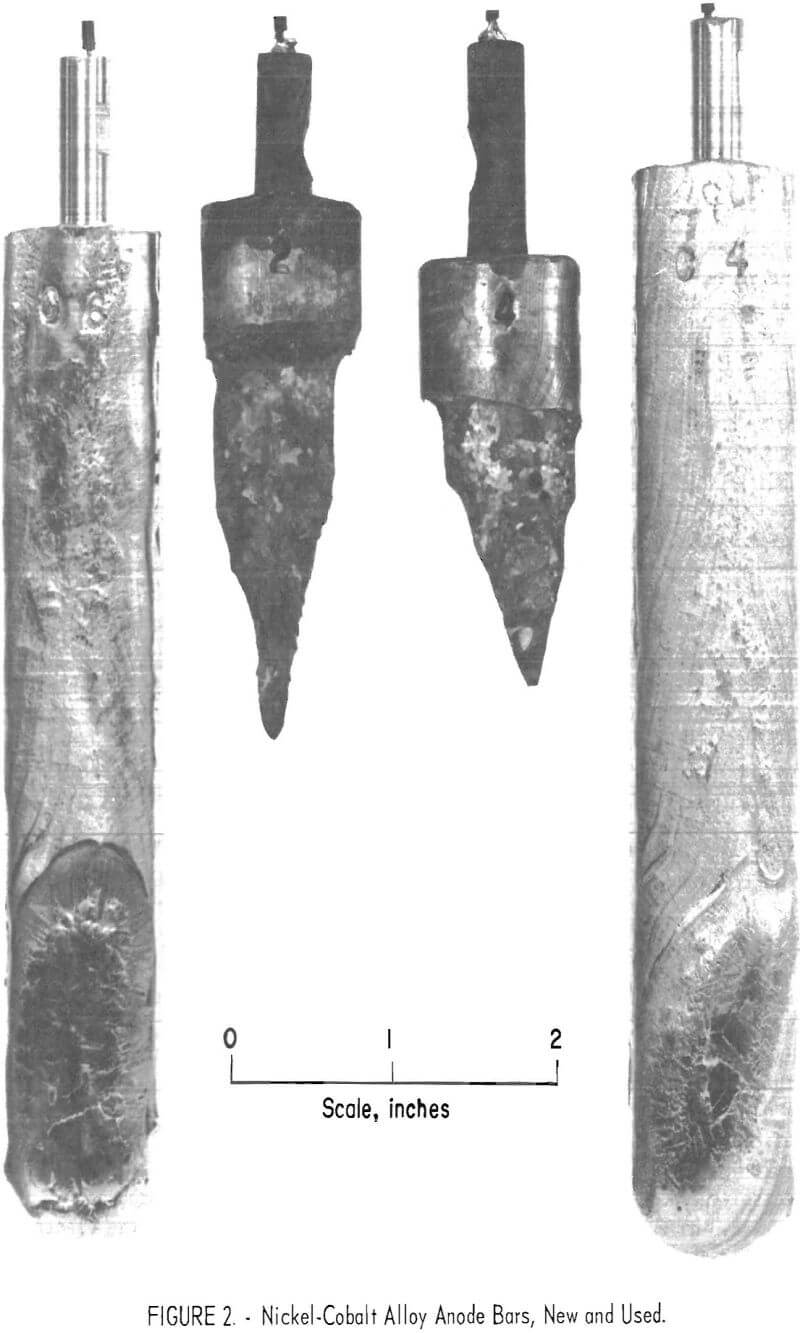
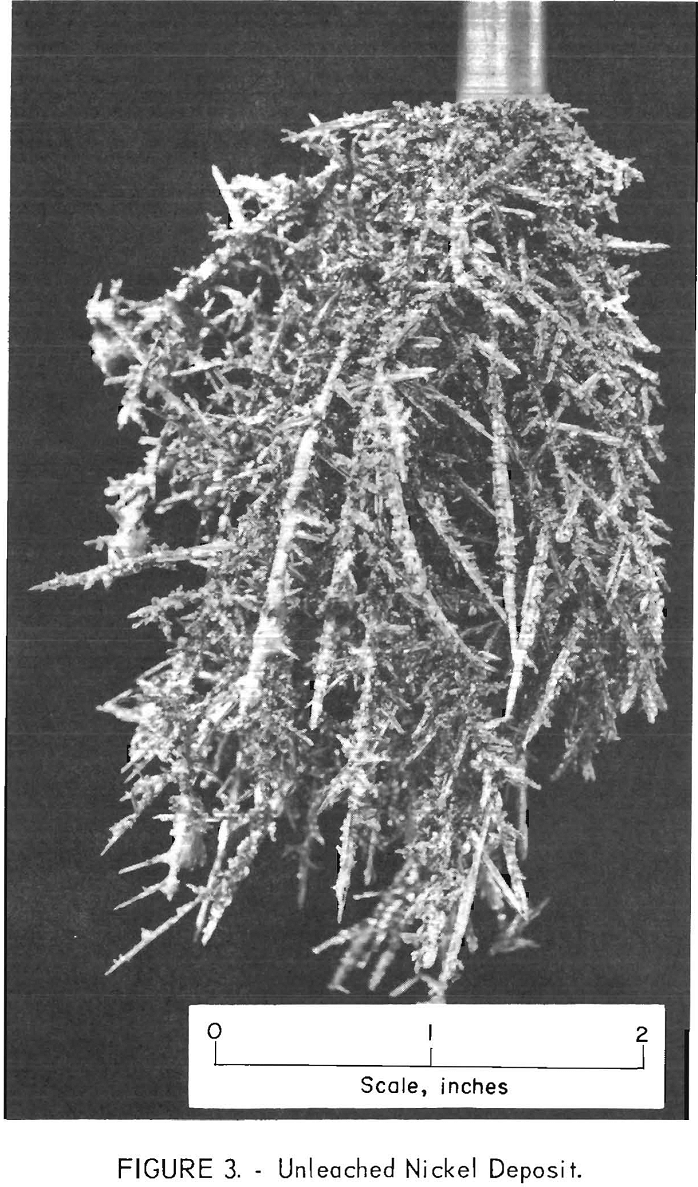
The selective electrodeposition of nickel using a molten KCl-LiCl-NiCl2 electrolyte and an anode of nickel-cobalt alloy was accomplished in a series of refining tests. A nickel-cobalt alloy anode containing 2.7 percent cobalt was used. This anode material and all others used in the investigation were prepared by arc-melting the desired amounts of nickel and cobalt into 300-gram bars. Figure 2 shows two new and two used anodes. Twenty-one tests were made in the series with approximately 40 grams of refined nickel deposited per test. The electrolyte contained 3,000 grams of the eutectic mixture of KCl-LiCl with 3.66 percent nickel as NiCl2. Figure 3 shows a typical deposit from this series. It weighed 41 grams, contained 0.02 percent cobalt, and was the 13th deposit of the series. It was made with an initial cathode current density of 210 amp/ft². The metal from individual tests ranged from less than 0.01 percent cobalt at the start, to 0.04 percent at the end of the series. The average cobalt content of all products was 0.02 percent for the entire series. During the series, the nickel content of the electrolyte dropped from 3.66 to 3.00 percent, while the cobalt content increased from 0 to 0.67 percent. This series of tests demonstrated that nickel and cobalt could be separated by molten salt electrolysis.
Factors Involved in the Selective Deposition of Nickel
Investigations were made to determine the factors that influenced the selective deposition of nickel from a nickel-cobalt alloy anode.
Cobalt Concentration of the Electrolyte
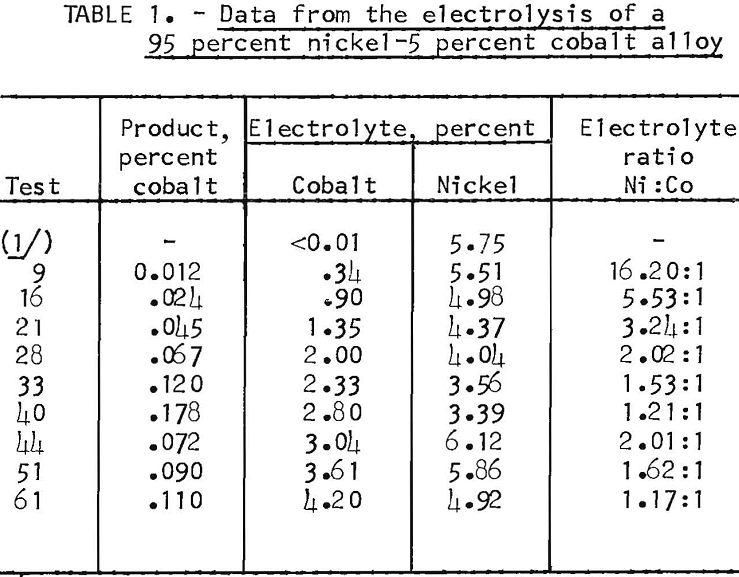
The effect of the cobalt concentration of the electrolyte on the selective deposition of nickel was determined in a series of tests. Approximately 40-gram cathode deposits of nickel were made in each of the 61 tests. Initial cathode current densities of 100 to 400 amp/ft² were used. Table 1 presents the data from representative tests in the series. The first eight nickel products contained less than 0.01 percent cobalt. In test nine 0.012 percent cobalt was found in the nickel product. Then, as the cobalt content of the electrolyte increased, so did the cobalt content of the nickel product increase. However, even with a 2-to-1 nickel-to-cobalt ratio in the electrolyte, the product only contained 0.067 percent cobalt compared with 5 percent in the anode.
To determine the effect of increasing the nickel concentration of the electrolyte after a substantial amount of the original nickel concentration had been replaced with cobalt, an addition of nickel chloride was made to the electrolyte after test 40. Enough NiCl2 was added to restore the nickel-to-cobalt ratio in the electrolyte to 2 to 1. The effect of the added nickel
chloride was to reduce the transfer of cobalt. The cobalt in the product of test 40 made with an electrolyte containing a nickel- to-cobalt ratio of 1.21 to 1, was 0.178 percent. The cobalt in the product of test 44, made when the electrolyte contained a nickel- to-cobalt ratio of 2.01 to 1, was 0.072. The amount of cobalt in the products after test 44 again increased. The rate of increase was not as great as for similar nickel-to- cobalt ratios from the first part of the series. The cobalt in the nickel deposit from test 61 contained less cobalt than for test 40, with a similar nickel-to-cobalt ratio. This showed that the selective deposition of nickel was dependent upon both the nickel-to-cobalt ratio in the electrolyte and the nickel concentration of the electrolyte.
Another series of tests was made to determine the effect of replacing essentially all of the nickel content of the electrolyte with cobalt. With an electrolyte containing 0.004 percent nickel and 4.76 percent cobalt, and a nickel cobalt anode containing 4.85 percent cobalt, the nickel products of two tests contained 4.42 and 4.48 percent cobalt. The transfer of cobalt was approximately equal to the cobalt present in the anode material.
Anode Dissolution
Anode current efficiencies in the series of tests made with a nickel-cobalt alloy anode containing 5 percent cobalt were always close to 100 percent. This meant that either both the nickel and cobalt were dissolving, or that the nickel was selectively dissolved leaving the cobalt. Inspection of the anode after each test indicated that the anode was dissolving nonselectively. There was no sludge or sponge residue left on the anode to indicate selective dissolution of the nickel. Analysis of the electrolyte showed that the cobalt content of the anode was appearing in the electrolyte. The cobalt content of the electrolyte gradually increased during the series of tests. Nickel was selectively deposited at the cathode. With an increase in cobalt concentration in the electrolyte there was a proportional decrease in its nickel content. Cathode current efficiencies generally ranged from 95 to 100 percent, which indicated that the excess nickel deposited at the cathode over the amount dissolved at the anode came from the nickel chloride content of the electrolyte. The loss of nickel from the electrolyte was balanced by the gain in cobalt. Thus, the dissolution of the anode was nonselective, with both the cobalt and nickel dissolving in the electrolyte.
Composition of Anode Material
The effect of anode material composition on the transfer of cobalt was determined by running successive series of tests, first with an anode containing 5 percent cobalt followed by a series with a pure nickel anode.
When the electrolyte reached a concentration of 4.38 percent nickel and 4.40 percent cobalt, a product that contained 0.19 percent cobalt was obtained. A pure nickel anode was then substituted for the nickel-cobalt alloy anode using the same electrolyte. Thirteen tests were then made with the nickel anode. The cobalt content of the products did not decrease, varying between 0.17 and 0.21 percent cobalt,. This signified that cobalt content of the anode was not a significant factor in the transfer of cobalt to the nickel product, except for its effect in increasing the cobalt concentration of the electrolyte. With an electrolyte composition that remained fairly constant, products were obtained that contained almost identical amounts of cobalt. Thus, a major factor controlling the transfer of cobalt to the refined product was the cobalt concentration of the electrolyte.
Effect of Cathode Current Density
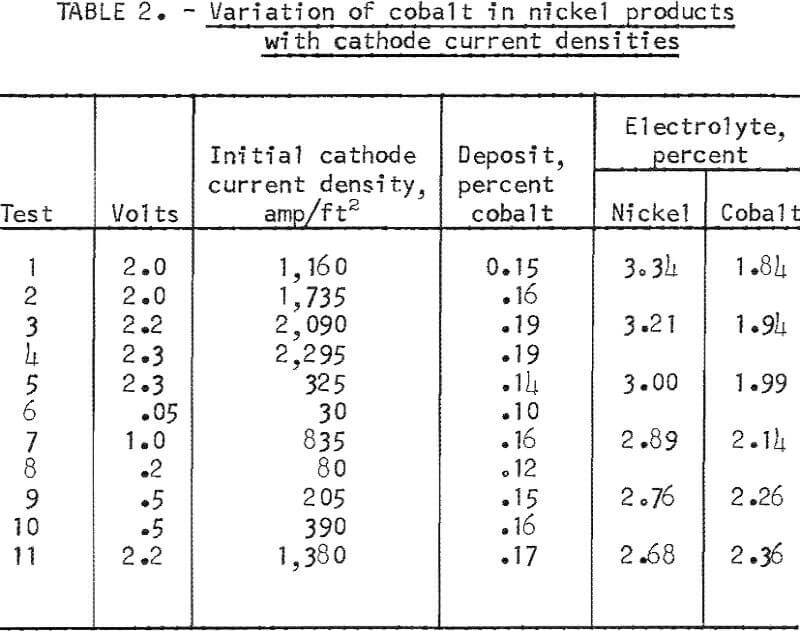
The amount of cobalt transferring to the nickel cathode deposit was influenced by the cathode current density used in electrolysis. The effect of varying the cathode current densities in consecutive tests is shown in table 2. The group of tests shown was made during a series using a nickel-cobalt alloy anode containing 5.5 percent cobalt. Electrorefining had resulted in a cobalt concentration in the electrolyte of 1.84 percent at the start of this group of tests. In general, the higher the current density, the greater was the transfer of cobalt to the product. The amount of product in each test was held at approximately 40 grams. The influence of current density on the type of nickel deposits is shown in figures 4 and 5. A cathode current density range of 200 to 500 amp/ft² was chosen as the most practical to use on the basis of both transfer of cobalt and deposition rate.
Recovery of Cobalt from the Electrolyte
The cobalt content of the electrolyte was recovered by first removing any NiCl2, in the electrolyte by electrolysis using a cobalt anode. When the cobalt had replaced the nickel content of the electrolyte, the cobalt anode

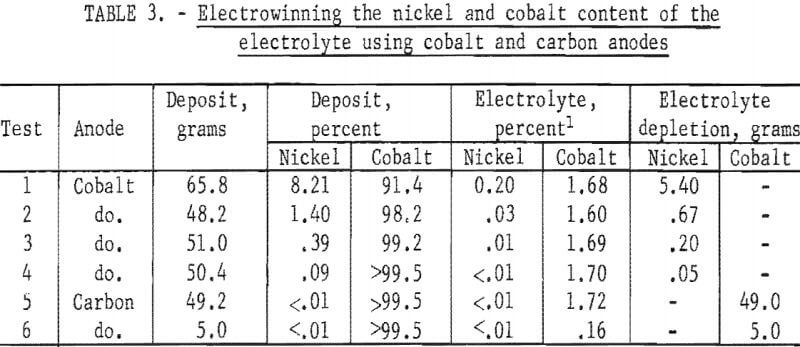
was replaced with a carbon anode and the cobalt was electrowon from the electrolyte. The results of one series of electrowinning tests are shown in table 3. The electrolyte contained 0.20 percent nickel (6.4 grams) and 1.68 percent cobalt at the start of the electrowinning series. The nickel was removed in four tests using a cobalt anode and a cathode current density of 200 amp/ft². The electrolyte then contained 1.72 percent cobalt (55.0 grams). Two electrowinning tests with a carbon anode and a cathode current density of 350 amp/ft² effectively removed the cobalt from the electrolyte. The final cobalt content of the electrolyte was 0.03 percent (0.96 gram) and 98 percent of the cobalt was recovered from the electrolyte.
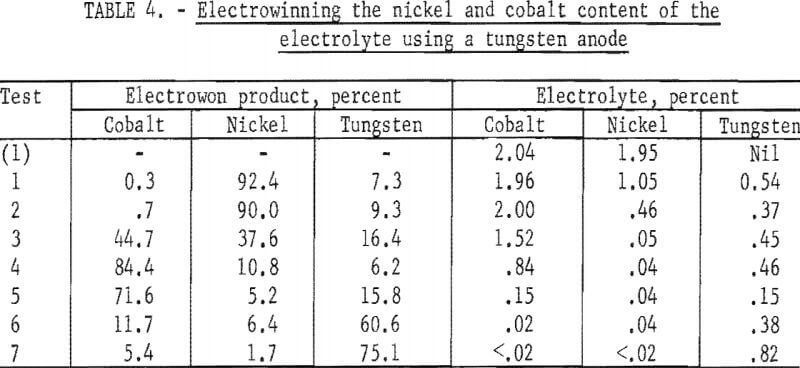
Several tests were made using tungsten as the anode material for electrowinning the nickel and cobalt from an electrolyte. The tests were not successful in producing a pure cobalt metal product after the bulk of the nickel had been removed. Tungsten dissolved at the anode and was recovered at the cathode with both the nickel-rich and the cobalt-rich products. Table 4 shows the results of a series of tests using a tungsten anode. The values shown for the electrolyte are for samples taken after removal of the electrowon products from each test. The possible use of such a method for producing tungsten-cobalt-nickel, tungsten-cobalt, or tungsten-nickel alloys was demonstrated.
High-Purity Nickel Preparation
While the primary objective of the investigation was the development of a process for the separation of cobalt and nickel, the investigation also demonstrated that a high-purity nickel could be obtained by electrolysis in a

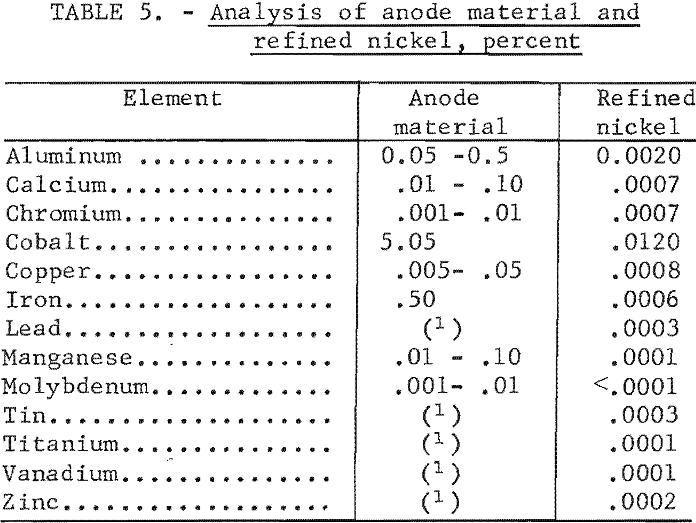
molten salt electrolyte. The analysis of the nickel product of test 9, table 1, along with the analysis of the anode materials, is shown in table 5. The analysis illustrates that a high-purity nickel was produced by a reduction of all impurity elements. In another series of tests using nickel shot as the anode material, similar results to those shown in table 5 were obtained on the refined material with the exception that no cobalt was detected in the refined product.
Electrolysis in Normal Atmosphere Cell
The use of a normal atmosphere cell for the separation of nickel and cobalt was investigated to compare with the results obtained in the helium atmosphere cell. Several advantages are possible by using a normal cell. The need for a pressure and vacuum tight system with slide valve would be eliminated. The equipment cost would be lower with an overall simplicity in construction and operation. Two main items that had to be determined were (1) whether contact of the hot electrolyte with air would result in loss of nickel chloride by oxidation, and (2) the effect of air on the hot cathode deposit on removal from the electrolyte.

A series of refining tests was made in the normal cell using a nickel-cobalt alloy anode containing 4.2 percent cobalt. Separation of cobalt and nickel was accomplished with results similar to those obtained on refining in the helium atmosphere cell. The major difference in the performance in the two types of cells was in a slightly greater loss of some of the normal cell electrolyte’s nickel content by oxidation or sublimation. With similar amounts of nickel and cobalt in the electrolytes, and with similar current densities, the concentration of cobalt in the nickel products from both cells was similar. A film of electrolyte on the metal crystals protected them from air oxidation on removal from the molten electrolyte. A typical unleached deposit from the normal cell is shown in figure 6. The major difference in the appearance of this deposit when compared with deposits from the helium atmosphere cell was that the electrolyte film on the surface of the nickel crystals was darker when cooled in air than when cooled in a helium atmosphere. On leaching, a small amount of nickel oxide was found in the electrolyte removed with the deposit. No oxidation of the metal crystals was found.
Comparison of Refining in Normal and Helium Atmosphere Cells
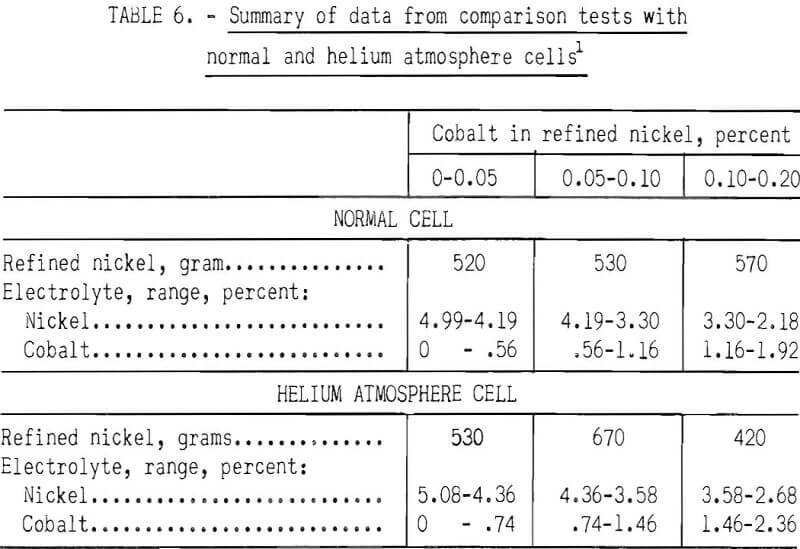
Two parallel series of refining tests were made to compare refining in normal and in helium atmosphere cells. Testing was conducted under similar conditions in each cell. Electrolysis was conducted at 450° C with initial cathode current densities ranging from 25 to 2,600 amp/ft² with potentials of 0.05 to 2.3 volts. The nickel-cobalt anode alloy contained 4.1 percent cobalt. The anode bars were similar to those shown in figure 2. Two anode bars were used with a single 7/16-inch-diameter cathode. The anode immersion was 4 inches and the cathode immersion varied between 1 and 4 inches, depending on the cathode current density desired. Five thousand grams of KCl-LiCi-NiCl2 electrolyte was used in each cell. The starting nickel content was 5.08 percent in the helium atmosphere cell and 4.99 percent in the normal cell. Approximately 40 grams of metal was produced in each test. Table 6 summarizes the performance of each cell. The table groups consecutive deposits into three groups which contained up to 0.05 percent, 0.05 to 0.10 percent, and 0.10 to 0.20 percent cobalt. Little difference was found in the refining in either type of cell. Electrolyte dragout was higher in the normal cell with a metal-to-salt ratio of 2.5 to 1, compared with 3.3 to 1 in the helium atmosphere cell. The average cobalt content of the nickel produced in the normal cell was 0.09 percent, and it was 0.08 percent in the helium cell. The nickel content of the helium atmosphere cell started at 5.08 percent and ended with a combined nickel and cobalt content of 5.04 percent. The nickel content of the normal cell started at 4.99 percent and ended with a nickel-cobalt content of 4.10 percent. The loss of nickel-cobalt chlorides from the normal cell was only slightly higher than from the helium atmosphere cell. In both cells, refining of nickel containing 4.1 percent cobalt to a product containing less than 0.20 percent cobalt was achieved.
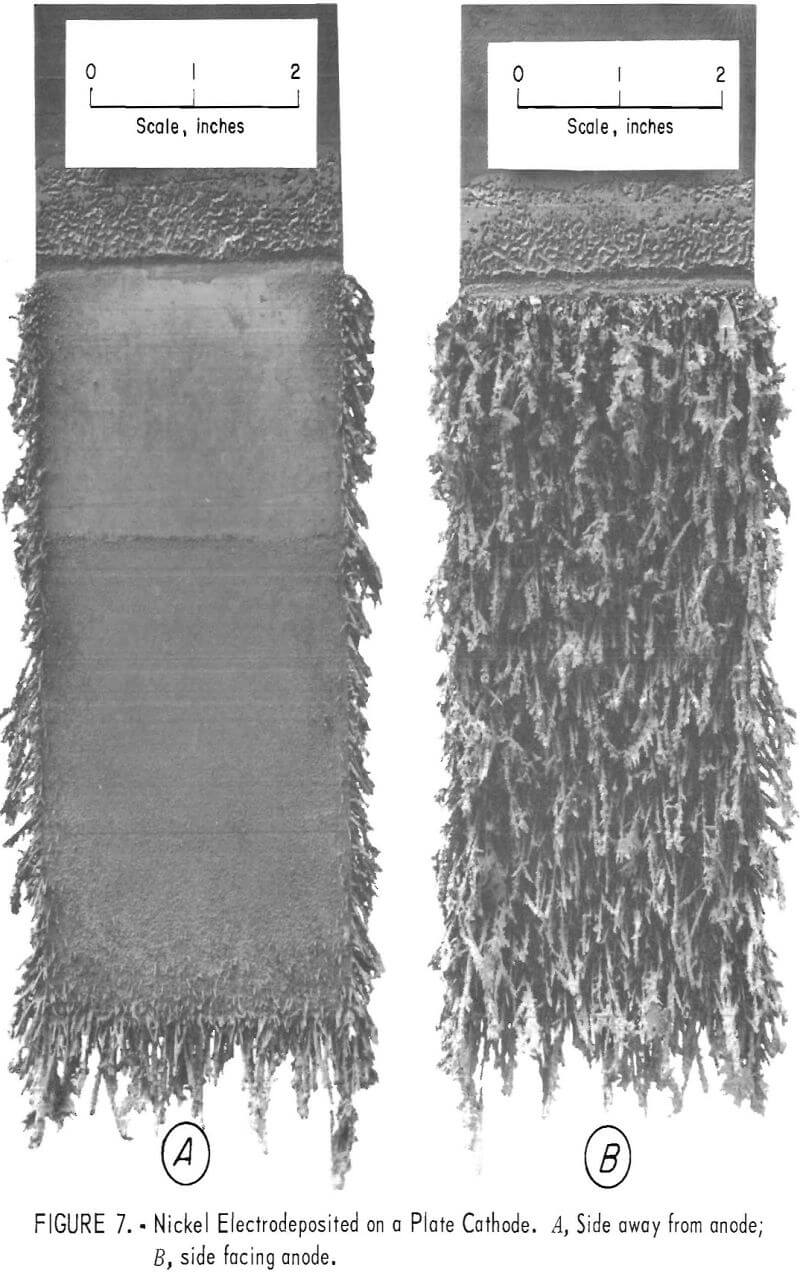
A deposition test was made in the normal cell using a plate instead of a rod cathode. Figure 7 shows the type of deposit obtained. The deposit was confined to the side of the plate facing the anode (fig. 7B). A cell utilizing plate cathodes would have to alternate anode and cathode plates.
