In an effort to evaluate the metallurgical results of an operating plant, 3 process stream samples (red points) provided were subjected to detailed modal analysis using protocols established for mineralogically similar ores. This method of analysis provides quantitative estimates of the mineral composition and of the mineral fragmentation characteristics. This data can then be used to evaluate the efficiency of the mineral separation process in the current plant circuitry. The plant was suffering unexpected Mo losses and this work allowed for better understanding and solutions.
The molybdenum content measured in the feed stream was approximately 0.10% of which 0.02% or 20% was determined by chemical methods to be in non-sulphide form. The sulphide minerals, which combined accounted for less than one percent of the ore mass were, pyrite, molybdenite and chalcopyrite respectively. The sulphide mineral suite was emplaced in a dominantly silicate rich host.
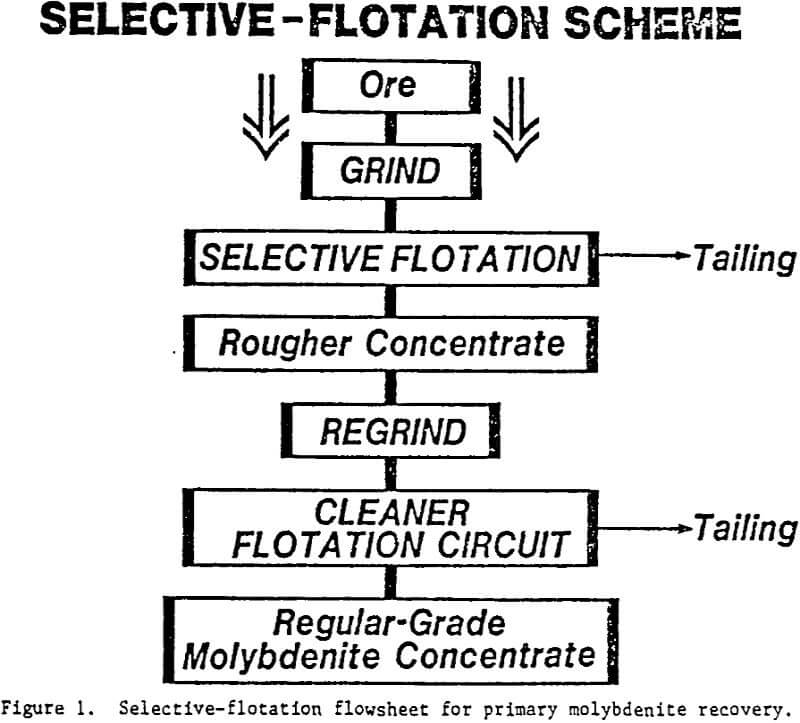
At a flotation feed sizing of about 135µm P80, the liberation of the molybdenite, when assessed in two dimensions, was 56%. When compared to similar ores now being processed worldwide, this level of liberation is sufficient to allow successful flotation recovery of most of the molybdenite bearing particles into a rougher concentrate. There is some evidence from mineral fragmentation studies, which suggests that still coarser flotation feed sizings could be used to achieve much the same molybdenite liberation levels; and hence, very similar metallurgical performances (Figure 1).
All of the unliberated molybdenite in the flotation feed stream was locked in structurally simple binary composites with non-sulphide gangue. Typically the majority of these binary composites contain very little molybdenite, thereby presenting an elusive target for the flotation process.
The final concentrate, which was sized at 55µm P80, was of relatively low grade assaying 42% molybdenum, or containing about 70% by weight molybdenite. The principal diluent in the concentrate was non-sulphide gangue, the majority of which was finer than 10 microns in size and were liberated when assessed in two dimensions. The copper content of the concentrate was about 0.45% copper, most of which was present as chalcopyrite, tetrahedrite∗ and enargite; trace quantities of bornite and chalcocite were also detected.
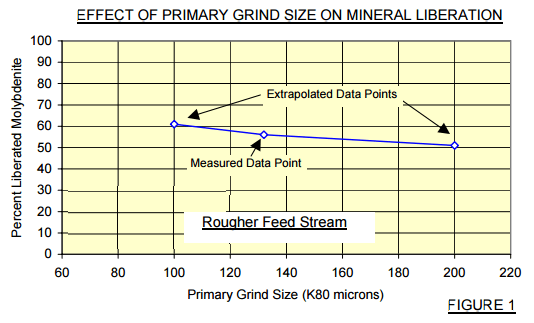
Liberated grains of molybdenite were 91% captured into the final concentrate. Approximately 11% of the molybdenite bearing binary composites were captured – predictably these composites contained significant amounts of molybdenite, probably accounting for their enhanced floatability. Figure 2 provides a synopsis of the recovery by particle size range plots of molybdenite bearing mineral particles in the current process. 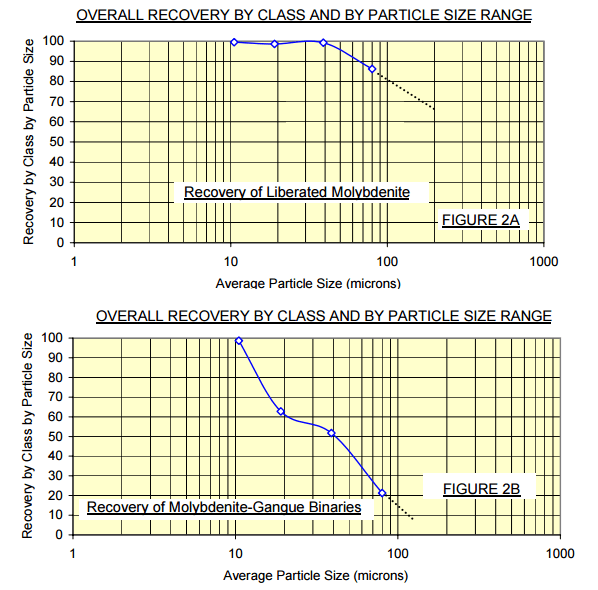
The modal analysis of the final flotation tailings stream revealed that about one quarter of the molybdenite was present as liberated grains. Most of these liberated molybdenite grains were sized finer than 10 microns. Of the unliberated molybdenite, all occurred as binary composites with non-sulphide gangue; the vast majority of these binary composites occupied the coarser particle size ranges in the tailings stream. The molybdenite losses to the final tailings stream at the time these samples were taken are summarized in the bar chart and tables of data displayed in Figure 3. 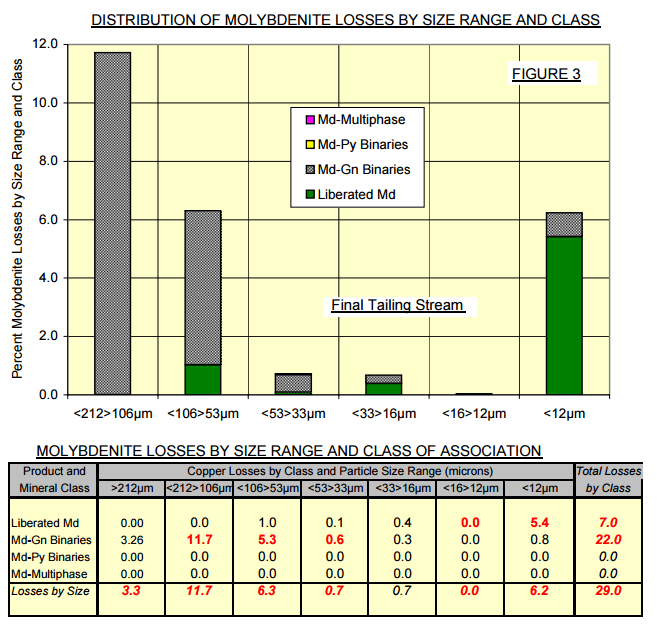 By using well dispersed, but standard molybdenite collectors, such as fuel oil or other non-polar collectors, flotation selectively could probably be maintained against pyrite and chalcopyrite while overall molybdenite flotation recoveries could be improved. It is probable that the fine liberated grains of molybdenite, now lost to tailings, would be preferentially captured by any change in operating mode. The very thinly scattered mineralized molybdenite bearing binary composites would be more difficult to recover under any circumstances.
By using well dispersed, but standard molybdenite collectors, such as fuel oil or other non-polar collectors, flotation selectively could probably be maintained against pyrite and chalcopyrite while overall molybdenite flotation recoveries could be improved. It is probable that the fine liberated grains of molybdenite, now lost to tailings, would be preferentially captured by any change in operating mode. The very thinly scattered mineralized molybdenite bearing binary composites would be more difficult to recover under any circumstances.
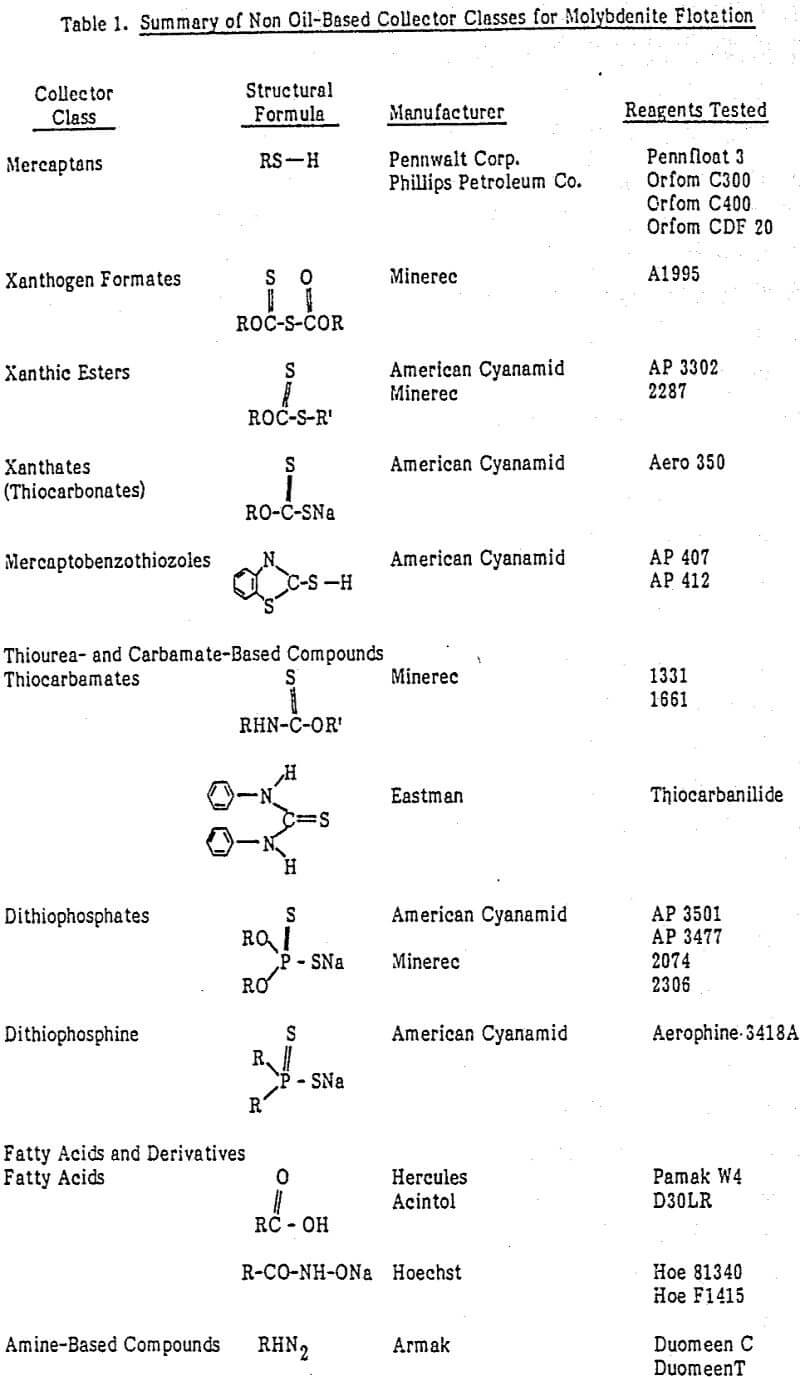
 Evaluation of Collectors for Molybdenite Flotation
Evaluation of Collectors for Molybdenite Flotation
Most primary-molybdenite producers use a selective-flotation process in which molybdenite is floated from the ore while other sulfide and non-sulfide gangue minerals are depressed in the same step. The process was developed at the Climax mill.
Standard laboratory rougher-flotation test procedure involved grinding a 2-kilogram ore charge in a rod mill at 60-percent solids. Reagents were added to the rod mill including a collector, syntex (0.01 lb/t), pine oil (0.06 lb/t), lime (to adjust pH to 8) and sodium silicate (0.30 lb/t). The ground pulp was transferred to a 1000-gram cell and flotation was performed at 35-percent solids and 1800 rpm using a Denver D-1 flotation machine. Concentrate was collected for a flotation time of 6 minutes.
Based on the criteria selected to evaluate the flotation data, the collector classes were categorized as having good, poor or no potential of being substituted for vapor oil in the molybdenite-flotation system. The results, summarized in Table 4, show that mercaptan, xanthogen formate, xanthic ester, and xanthate collectors exhibited good potential as substitutes for vapor oil. Mercaptobenzothiozoles, thiourea- and carbamate- based compounds, dithiophosphates and dithiophesphines showed poor potential as collectors. Fatty acids and their derivatives and amine-based compounds acted as depressants for molybdenite flotation under the conditions studied, as the molybdenite recoveries obtained with them were lower than those obtained in collectorless flotation.
Molybdenite concentrate grades obtained with the collectors exhibiting good potential as substitutes for vapor oil were significantly lower than those obtained with the vapor oil or collectorless notation. The production of lower-grade molybdenite concentrate results because these collectors recover significantly more pyrite and other sulfide-bearing metal values.
Mercaptans, xanthogen formates, xanthic esters and xanthates give reasonably good molybdenite recoveries as compared to vapor oil in the molybdenite-flotation system.