Table of Contents
The American Iron and Steel Institute (AISI) has conducted major cooperative research efforts with the United State Department of Energy (DOE) and industrial organizations since the autumn of 1988 on the development of a process for direct steelmaking. This research has been inspired by the fact that the direct iron reduction smelting process holds several advantages over conventional coke and blast furnace process. These include: 1) the use of more desirable coal rather than coke, 2) a higher smelting intensity and productivity, 3) the possible use of ores and concentrate directly for smelter feed rather than sinter and pellets, 4) lower capital costs, 5) lower operating costs, and 6) the economical feasibility of small scale plants.
AISI-DOE Direct Ironmaking Pilot Plant Design and Process
The AISI-DOE Direct Steelmaking Process has been described by Aukrust. It uses bath smelting of unreduced and partially pre-reduced iron oxide pellets with coal, oxygen, and flux in a

vertical vessel. The current vessel has apaproximate outer dimensions of 14′ tall and 9′ wide (Figure 1). It can smelt up to 10 tons of hot metal per hr. The vessel wall is lined with refractory bricks with water cooled plates. The estimated variable operating cost of the ironmaking process is a low $160 per annual ton for a plant with a hot metal capacity of one million metric tons per year.
The process is initiated by melting thirteen to fourteen tons of iron in an induction furnace. The molten iron is then placed within the pressurized smelter where it provides the energy to propagate a continuous reaction between oxygen and coal. This internal reaction continues as long as the raw materials, coal and oxygen, are replenished and supplies all of the necessary energy to pre-reduce, melt, and reduce the iron oxide. The coal also provides a source of carbon to reduce the iron oxide into iron.

Cyclone Dust
The cyclone dust samples were examined to determine the particle size distribution and the mineralogy of the particles. The mineralogical form of the sulfur present is of special interest because it is desired to feed the dust back into the furnace rather than experience the cost of removing it as waste. The sulfur content of dust formed from the treatment of iron ore pellets ran about 2%, and that amount of sulfur inhibits the recycling of the cyclone dust. Knowledge of the mineralogical form of the sulfur would aid in designing procedures for removing part of the sulfur from the dust.
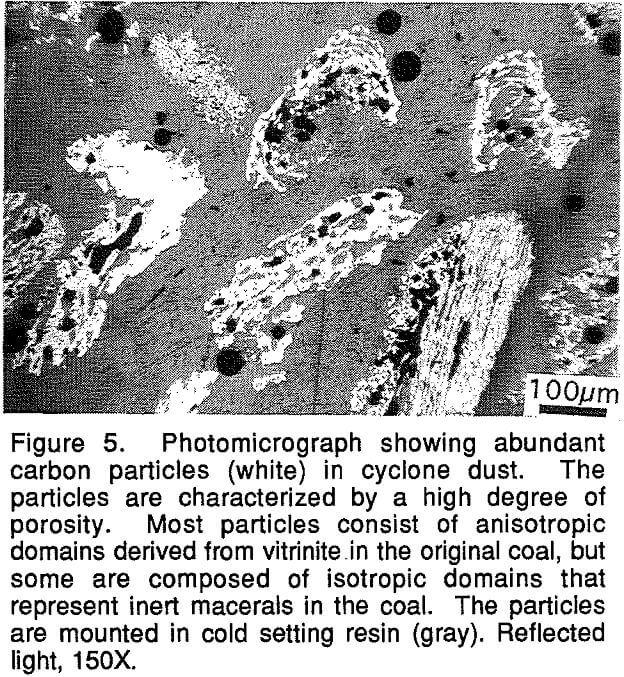
The only sulfur-bearing phase found in the dust samples examined was pyrrhotite. No sulfur was detected by SEM-EDS in the carbon particles.
Sludge
The sludge samples are collected from the gas by means of a scrubber. The wet sludge was dried, cleaned with acetone, and mounted in polished sections for microscopic study. Reflected light and SEM studies were conducted on the sludge and cyclone dust samples.
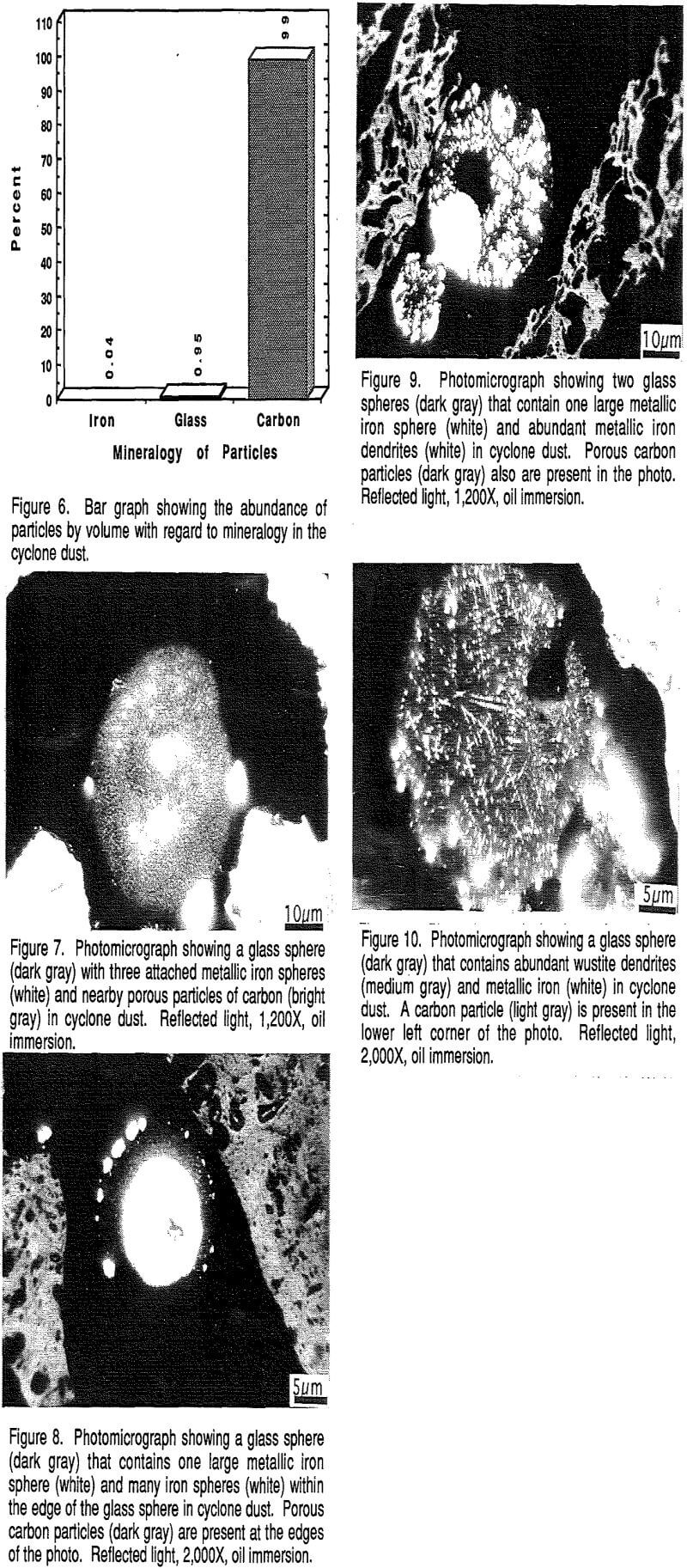
The results of counting of 500 particles under reflected light to determine the range and abundance of different particle sizes are shown in Figure 18. The figure shows that 69% of the particles occur in the <2.5 µm size fraction, and that the majority of the remaining particles fall in the 2.5-5 µm (21%) and 5-25 µm (9%) size classes.
Build-Ups
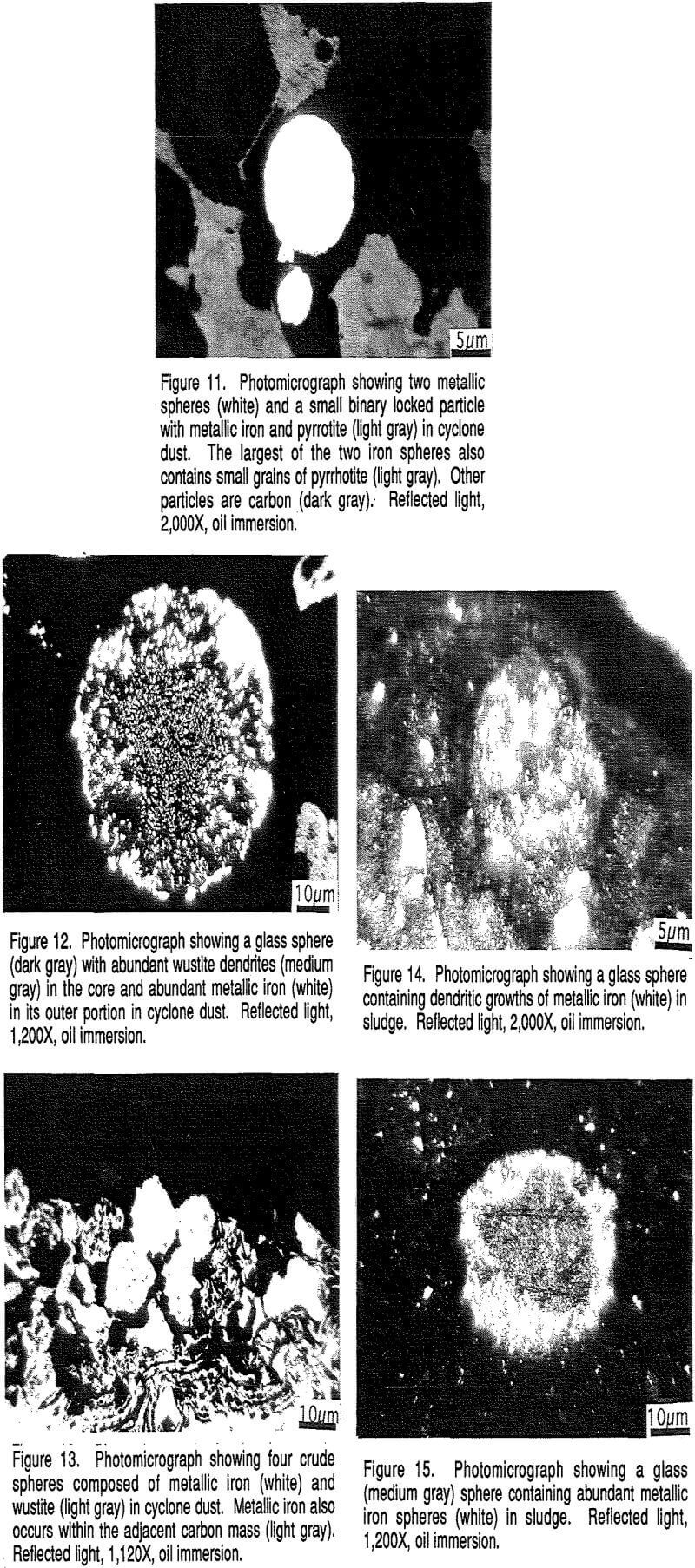
The build-ups are typically grown together to form solid masses that can fill as much as 30% of the off-gas duct. Such build-ups are deleterious in that they restrict the offgas flow. Their mineralogy and textures have been investigated to determine the composition of the build-ups and to evaluate their method of formation.
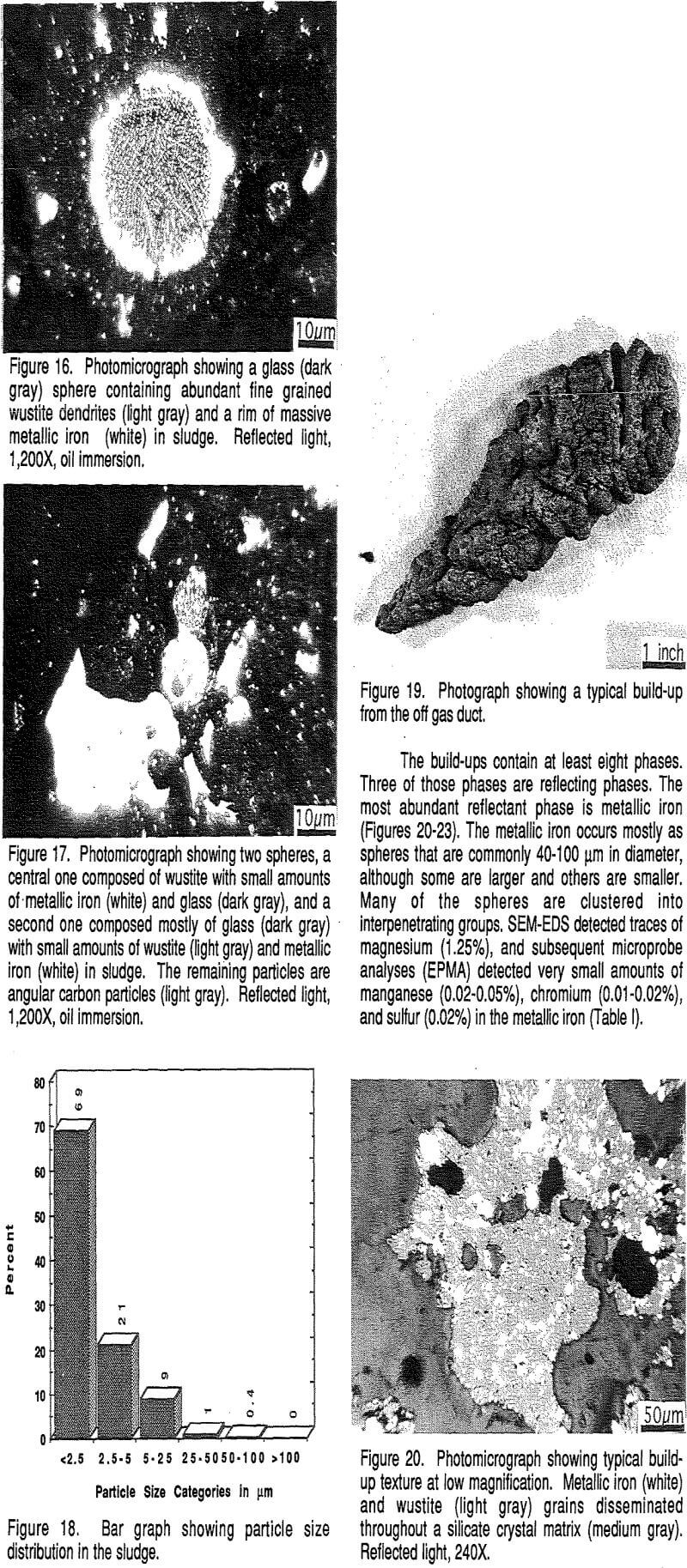
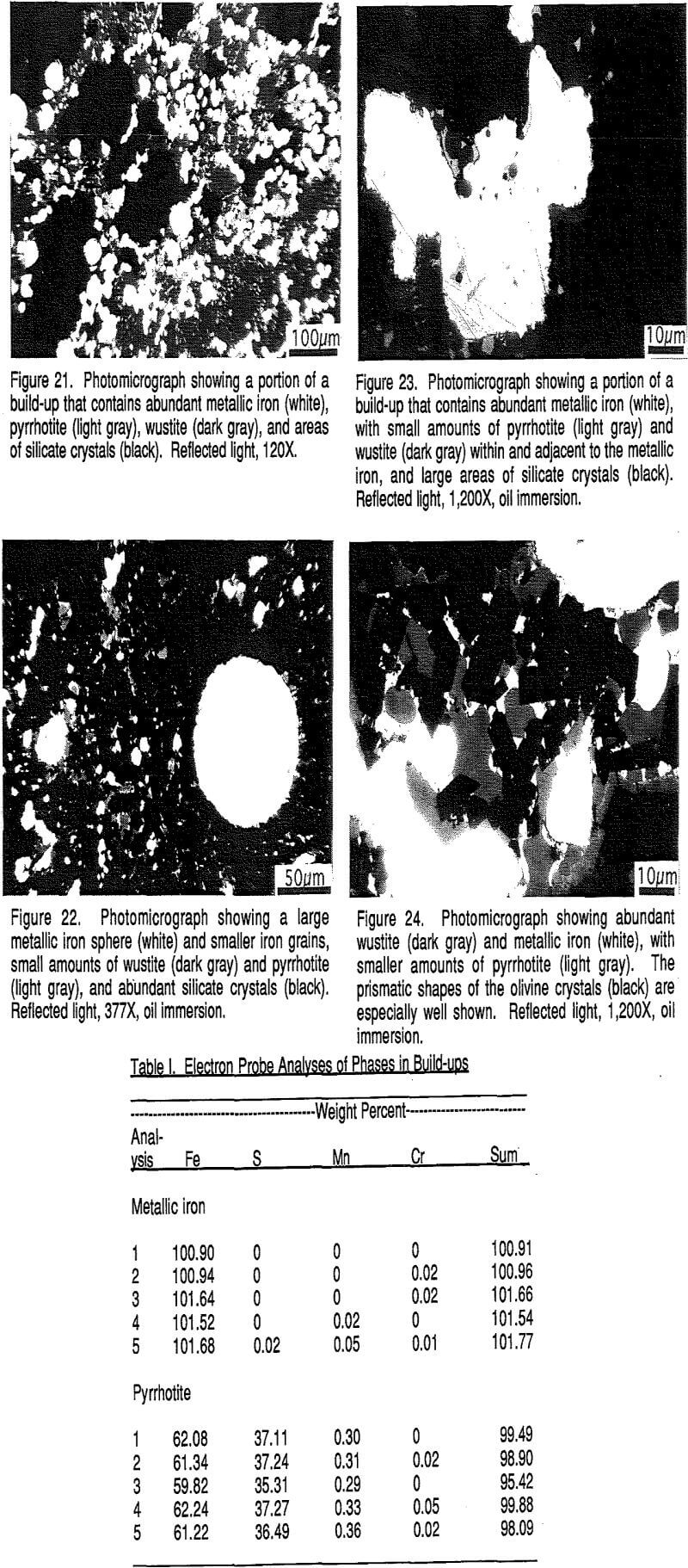
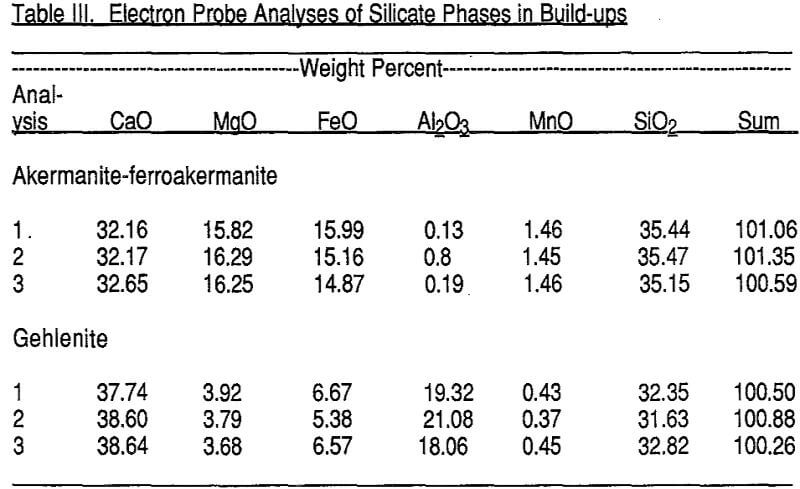
Wustite (FeO) is the next most abundant phase. Wustite may form oxidation rims around or partly around the metallic iron spheres, but more commonly it forms its own grains, about 20-30 µm across, that are single or clusters of grains adjacent to metallic iron grains or contained within non-reflective phases.

The above four reflective phases, metallic iron, wustite, pyrrhotite, and magnetite (?) constitute between 20% and 40% of the build-up materials, varying from place to place.

Summary of Build-up Character
The build-ups consist mainly of crystallized olivine, akermanite-ferroakermanite, gehlenite, and spinel, together with rounded blebs of metallic iron that congealed from the molten state and closely associated wustite. The textures shown by the build¬up materials are not those that would be expected from simple agglomeration by bonding. That is, none of the materials exhibit textures like those shown by magnetite-hematite pellets. Instead, the build-ups exhibit massive textures with intergrown crystals and grains. The silicate crystals appear to have crystallized in place.