Table of Contents
The term refining has been very often applied to the removal of base metals from the noble ones, or, in other words, separating the oxidizable from the non-oxidizable ones. When used in this sense it is generally applied to the term bullion, which is an alloy carrying gold and silver, the bullion being pure when it contains gold and silver only. A special term, parting, is borrowed from assaying, and means the separation of gold from silver. In the foregoing processes it is hard to. draw the line where one begins and the other ends, but the term refining is used generally here to indicate the separation or partial separation of whatever metals are present with gold.
In order to purify gold and remove the silver from it by any process analogous to the cementation with nitre or salt in a reasonable time, the alloy must be in such a fine state of division that the silver will be removed almost instantly, or diffusion must be assisted by actually keeping the alloy in a molten state. Nitric acid, the active ingredient in the first cementation process described, would not be effective in this case, since even if nitrate of silver did form, the temperature is so high that it would be decomposed into oxide of silver, and finally metallic silver and oxides of nitrogen. In fact, nitric acid itself would not be stable at this temperature.
The second active agent was chlorine, or hydrochloric acid, and as the former is known to be much more active than the latter, a process of refining by this means was devised by Mr. Lewis Thompson in 1838. The alloy was melted in a vessel and a stream of chlorine allowed to pass over it. The silver was rapidly converted into chloride, and the gold was thus rendered pure. Later experimentalists found that there was no volatilisation of gold in this process, and that the addition of a chloride of the alkalies or alkaline earths prevented the volatilisation of the chloride of silver formed. It is somewhat difficult to believe that no gold volatilises during such an operation, for if pure gold is heated in a current of chlorine, yellow feathery scales of the chloride will sublime on the upper part of the tube some distance from the flame. This volatilisation is not appreciable until the silver has been removed.
Nearly 30 years after Thompson’s discovery, Francis Bowyer Miller, an assayer in the Sydney Mint, discovered and perfected what is known now as Miller’s process. He found that by passing chlorine gas into the molten alloy that practically the whole of the chlorine first passed in united with the silver, which in this way could be almost perfectly removed from the bullion as chloride. By passing chlorine over molten alloy the silver would be slowly removed, but most of the chlorine would escape unaltered; the chloride of silver coat also would prevent contact after a short time.
It was assumed by Miller that chloride of gold would not form at the temperature of molten gold, since the compound is decomposed at a dull red heat, but although this assumption was incorrect, the operation of passing chlorine through the molten gold does not allow of volatilisation of the gold while silver is present. The covering of borax and chloride of silver also appears to minimise, if not prevent, this loss.
The process was so successful that it has been adopted at all Australian Mints. Before Miller’s invention, the gold coins made at the Sydney Mint were alloyed with silver, since the cost of removing this and replacing it by copper was more than the value of the contained silver.
The following particulars of the operation at the Melbourne branch of the Royal Mint are partly taken from an account supplied to T. Kirk Rose by Mr. Francis R. Power, and partly from inspection of the process, and particulars kindly supplied through Mr. Wardell, the Deputy Master.
Bullion is received at the Royal Mint in many forms. It comes as alluvial gold, admixed with impurities, as retorted gold, and as smelted gold, more or less admixed with other metals. All classes of bullion are melted with the object of obtaining a sample, the assay of which will accurately represent the bar. Ordinary samples are melted with a flux consisting of:
100 parts fused borax
50 soda carbonate
10 nitre.
If the gold is very impure more nitre is added to this flux, a sample is taken to check the subsequent toughening, which is done either with chlorine or nitre until sufficient of the base metals is extracted to enable samples representative of the mass to be taken. Since after weighing and assaying any particular bar loses its individuality, all that is aimed at in the preliminary partial refining is to eliminate any metals or elements which prevent the bullion from being homogeneous, no gold and no silver is removed by this operation.
Equipment and Supplies
Furnaces
The furnaces used for melting the gold are shown in section in Fig. 1. They are cylindrical instead of the usual square sectioned ones, since this form is more economical in fuel, and are more readily cleaned from adhering clinker. They are 12 inches in diameter, and 21 inches deep. Five fire bars, 1½ inches square, and 18 inches long, rest in a cast-iron frame, D, 12 inches by 2 inches, and are supported by a bar at the back. These bars ere six inches above the floor. The ash pit, F, is a cast-iron box, below the level of the floor, and the draught passes through a grating at floor level covered with an adjustable damper plate, M. The escaping gases pass through the flue L, which leads to a series of condensing chambers, thence to the stack, which is 80 feet high. The furnace itself is built of arched fire bricks, B, 9 inches by 4½ inches, tapering from 2¾ to 2 inches. These are set in an iron cylinder, A, 21½ inches in diameter, and 3/8 inch thick. The cylinder rests on an iron plate, C, 5/8 inch thick, and 22 inches in diameter, with a 12 inch hole in the centre. This plate is supported on brickwork. The vertical cylinder is surrounded concrete rammed in, N. When this is set the facing plate is moved out 2 or 3 inches, thus providing an air jacket between the concrete and the external plate of the furnace, keeping the latter cooler. The upper surface of the furnace is also protected by a cast-iron plate. The furnace cover consists of three fireclay tiles, two being 20 inches by 6 inches, the third being smaller—all are bound with iron bands. The middle one is perforated with a inch hole, through which the chlorine delivery pipe passes.
The Crucibles and Connections
The pot in which the gold is melted prior to passing chlorine through is made of clay of fine texture, similar to French clay. These are 10½ inches high, 5 inches in diameter, 3/8 of an inch thick at the top, and gradually increasing to 1 inch at the bottom. These pots are fitted loosely into a guard pot for safety; the guard pot is a plumbago crucible, 8¼ inches high, 6 inches internal diameter, 5/8 inch thick at the top, and ¾ of an inch thick at the bottom. This stands in a cylindrical fire brick 5 inches in diameter, and 2½ inches high. Fire-clay lids, dished to catch any gold projected by too rapid a current of gas, and having a slit in them to allow of sliding them over the pot without shifting the chlorine pipe are provided.
The, pipe stem is 24 inches long, tapering from 3/8 to ½ an inch at the end inserted into the gold, and is wedge-shaped to facilitate the escape of the chlorine when resting on the bottom of the pot. The bore is 1/8 inch in diameter. The thin edge of the pipe stem is attached to the branch delivery pipe by a piece of ½ inch rubber, about 2½ inches long, which connects with an ebonite junction, G, 3 inches in length, with a bore of 1-10 inch, turned with a ring round the middle, which acts as a rest for the 8oz. weight, H, used as a sinker for the pipe stem. One end of the ebonite junction is ½ inch in diameter, the other ¾ inch, the latter being connected by a stout rubber tube 3 or 4 inches long, to a 14 inch lead pipe, ½ an inch in diameter, which is connected by a rubber junction to a glass stopcock, I, from the spigot of which a ¾lb. lead weight, J, is suspended to prevent the pressure of gas from blowing it out.
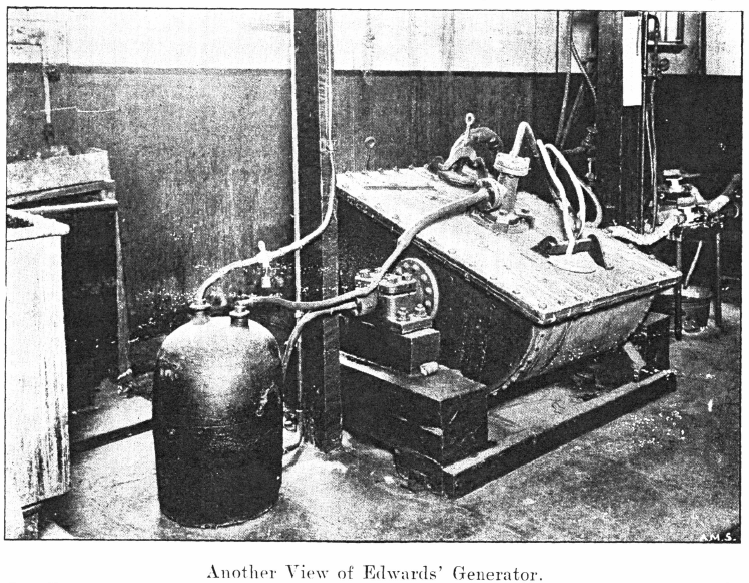
The Chlorine Generators
The jar generators described by Dr. Rose have been thrown out, and two Edwards’ Patent Chlorine Generators installed.
These consist of semi-cylindrical vessels hung on trunnions.
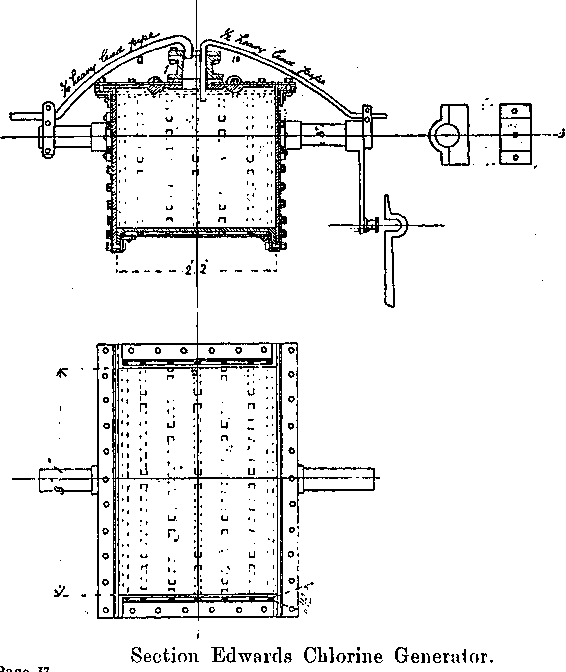
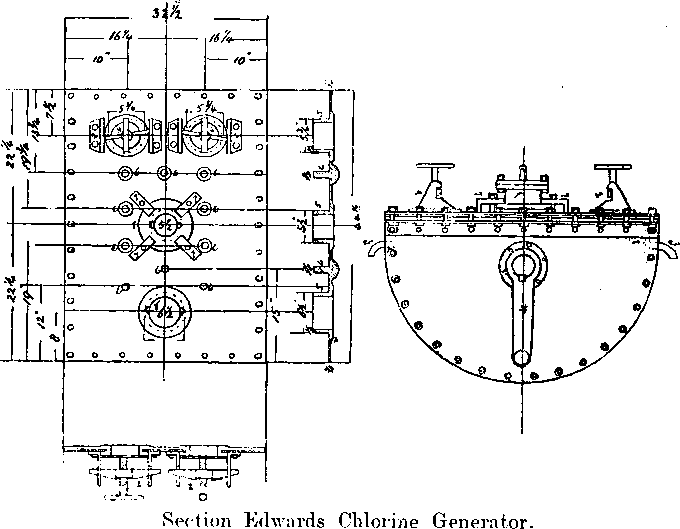
The vessel is made of boiler plates riveted together, and lined with sheet lead. The lead is corrugated below so as to give greater strength, but is smooth inside, steam is admitted through a pressure regulator through a trunnion on one side, and fills the space between the lead and iron, thereby providing a steam jacket, the pressure, and therefore the temperature of which is controlled by the regulator; any condensed steam is drained off by means of a pet cock provided; steam escapes through a pipe leading through the other trunnion.
One comer of the generator is attached by means of a connecting rod to a crank which gives it an oscillating motion, thereby preventing the chemicals from packing or becoming baked on the hot lead surface. Holes about 6 inches in diameter left in the flat cover of the generator. These serve for the introduction of the manganese dioxide and acid, and also for washing out the salts. After the charge is exhausted, lead¬faced, flat iron covers are removed: these are readily fastened in position by the pressure of a screw bolt at their centre. The screw passes through a horizontal bar, the ends of which slip under projecting lugs riveted on to the generator. The acid is supplied through a siphon pipe near the centre of the flat surface of the generator; the longer limb being sufficient to prevent the acid being thrown out by the internal pressure of the gas.
The chlorine escape pipe passes through the cover, and is connected with a fixed leaden pipe at a point opposite one of the trunnions, so that the oscillation of the generator will only give a minimum movement at this point.
The charge used consists of 100lb. manganese dioxide 130lb. salt 275lb. sulphuric acid.
The two generators are used alternately, except when it is required to refine a large amount of gold; in such a case the two generators are used together. When the charge is exhausted in one generator it is connected to the second, and the chlorine gas still remaining is displaced by gradually filling the vessel with water. One of the cover plates is then removed, and the charge is emptied into an underground drain.
The gas passes through a pair of earthenware jars provided with two necks, in which many of the acids or salts mechanically carried over are retained. From these the gas delivery pipe leads to a distributing vessel with two necks, and partly filled with manganese chloride solution. A pressure gauge of 1 inch glass tube 15 feet high is luted to the bottom of this vessel, and fixed to the wall by brackets. Since one inch of gold will balance a column of about 19 inches of water, the liquid in this tube must be from 10 to 11 feet in height to force chlorine through the 7 inches of gold in the pots. The pressure exerted is about 5lb. per square inch. A four-way tube of lead or pottery is pressed through the second neck of the vessel, and each arm is connected by thick rubber to glass stopcocks to which ½ inch lead pipes are joined, these pipes leading to sets of four and five furnaces.
When the flow of chlorine through the gold is stopped the chlorine escapes through a safety pipe. This is provided by having a two-necked earthenware vessel containing such a quantity of water that when the pressure of the gas exceeds the working pressure required, the end of a glass tube, passing to the bottom of the vessel, and connected above the neck with a 4 inch lead pipe ten feet high, becomes unsealed, and the gas escapes through the water in large bubbles, passing thence through a glass pipe inclined at an angle at the top of the lead pipe into the air. When sufficient gas has escaped to reduce the pressure to the working limit the pipe is automatically sealed.
All rubber junctions are covered with calico, and painted, and where practicable secured with copper wire.
Passing the Gas Through the Molten Metal
The guard with the clay liner is placed in the furnace, and 2 or 3 ounces of fused borax added. It is heated until dull red. The ingots are then added, the weight of these being about 700 oz. Fuel is added, and the dampers opened. As soon as the gold is melted, the lid is put on and the pipe stem, carefully annealed and heated to bright redness, is pushed to the bottom of the pot, chlorine at the same time being gently turned on to avoid the plugging of the tube through the gold solidifying in it.
The supply of chlorine is adjusted so as to avoid projection of globules. This can be told by feeling the pulsations of the rubber—when the gold contains much silver or base metals the absorption of the chlorine takes place rapidly but quietly, very little motion of the molten metal being apparent, but near the end of the operation the gas must be admitted in a fine stream only. The chlorine is not dried before it passes into the gold, but the small amount of water vapor present does not affect the operation. It was formerly the practice to dry the gas with strong sulphuric acid, but this has been abandoned as unnecessary.
The order in which the metals chloridise has never been determined. Iron appears to come off separately, and is attacked at the commencement, while silver and copper remain and come off practically together.
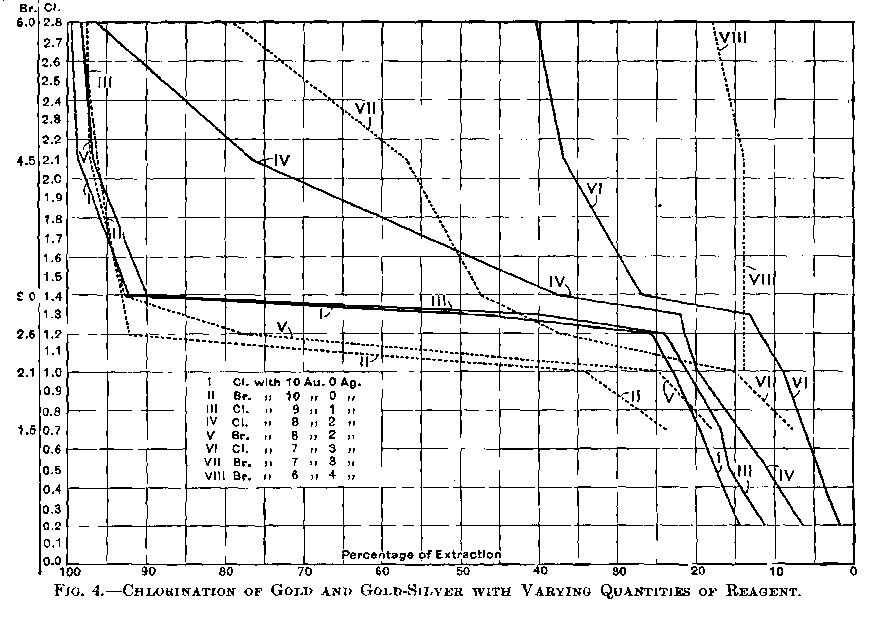
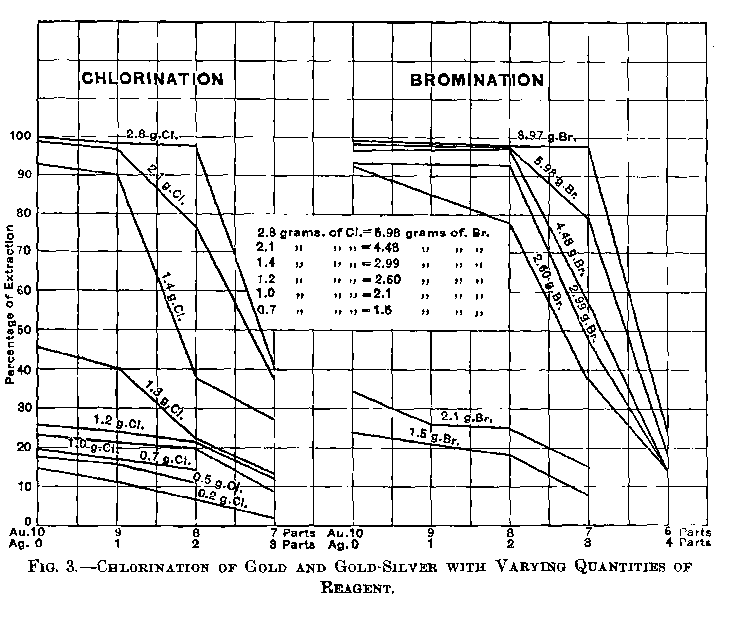
When the bullion contains much base metal the consumption of chlorine is greater, and the time occupied longer, but not in proportion to the amount contained, since a more rapid stream of chlorine can be safely admitted than when the base metals and silver are nearly all removed.
The usual time allowed is four hours for a pot containing about 700oz. of bullion. If the bullion is nearly fine a much shorter time would suffice; for instance, 2 per cent, of silver and 0.5 per cent, of base would take about 1½ hours; 3.5 of silver and 1.5 of base, two hours.
Owing to the presence of air at first in the chlorine mains the stream admitted is slow at first, otherwise there is danger of spitting.
When the operation is nearing completion the flame issuing from the holes or slit in the lid becomes small, and alters in appearance; it becomes very luminous, has a brown edge, and if a white, rough, cold surface is plunged into it will become coated with a yellowish-brown tinge. It consists mainly of ferric, chloride, with traces of silver chloride and gold. As soon as this stain appears the current of gas is reduced, and allowed to pass in for another 15 minutes, when the clay pipe is withdrawn, and the clay pot lifted out of the guard pot. The pot is allowed to stand under a hood to carry off the fumes until the gold has solidified. The liquid chloride and fluxes are then poured into a mould provided with a hood. The pot is then broken, and the cone of gold dropped into the guard pot, and cast into two flat ingots, 12 by 4 by 1½ inches. These bars, while still hot, are dropped in dilute sulphuric acid, and then water, and are still hot enough to become dry.
A modification of the process has been introduced. After the chlorine has been passed into the bullion for some time the chloride of silver which forms occupies twice the space that the silver did, and rises in the pot. When much silver was present the chloride was ladled out from time to time, to prevent it overflowing, and poured into a mould on top of the furnace. This practice has now been adopted for all the bullion. The chloride is baled out in such a way that any drip falls back again into the crucible. When the chloride film becomes thin some gold is also picked up—the last pourings are, therefore, put into a separate mould, when the gold present solidifies, the chloride is poured off, and the gold returned to the crucible. The last portion of silver remains on top of the gold, bone ash is added to thicken it, after which the refined gold is stirred and poured, the pot being returned at once to the furnace to be used for a fresh lot of bullion.
The chlorides contain 5 to 10 per cent, of gold in feathery particles. Generally speaking the gold is not present as chloride when the chlorine is passing, but chloride of gold has been found in the silver chloride when the process has been experimentally carried too far. The chlorides are supersaturated with chlorine and this is copiously evolved on cooling. To recover this gold 7 per cent, of their weight of bicarbonate of soda is added cautiously and without stirring, which produces a shower of globules of reduced silver, and these, falling through the chlorides, carry down nearly all the gold; another lot is needed to carry the balance down. The pot is lifted out, the button allowed to settle and solidify, when the chlorides are poured into a mould 12 by 10 by 2 inches. The silvery button obtained contains from 40 to 60 per cent, of gold. The gold in bars and in the buttons contain 99.85 per cent, of the gold issued for refining, the balance being practically in the pot. The maximum quantity left in the silver is 1 part in 10,000, but it is usually from half to one-third of this quantity.
The cakes of impure chloride of silver are sewn up in coarse flannel bags and boiled with water in a wooden vat for four or five hours. The salt present in the cake helps in the solution of the cuprous chloride present. The cakes are placed ultimately with wrought plates 1/8 inch thick, in a cast iron tank lined with similar plates. The plates are prevented from touching the bags by means of laths of wood, otherwise copper would be reduced in the bags and would be difficult to separate from the silver. The reduction is slow on starting, unless either some liquid is left from some previous operation or some chloride of iron is added. The bath must be heated by a jet of steam and kept boiling from two to four days. When the operation is complete no hard lumps can be felt in the bag. The silver is reduced in this way while the copper is not. When reduction is complete the silver is washed and smelted without fluxes, the average grade of the metal so produced being about 980. The total loss of silver amounts to about 1.6 per cent.