Table of Contents
Mercury or quicksilver fed into stamp mortar-boxes and riffles or applied to copper plates has been for ages the method of catching free gold. Many millions of ounces of gold have been saved thereby, and the mercury has been returned to circulation with small loss. Amalgamation is still practiced, but the recovery of gold by traps, jigs, cells, and cloth is lessening the amount so saved, although gold from these appliances is amalgamated for cleaning up.
Amalgamation is affected more or less by some pyrite minerals, especially if decomposed, also by antimony, bismuth, and copper.
If such is the case, tabling could precede amalgamation or corduroy should be substituted.
Mortar-box. If a mine has a stamp-mill and inside amalgamation is desirable, mercury is added to the mortar-box in quantity enough to form a spongy amalgam therein and not enough to work out on to the copper plates and make them sloppy with mercury.
Copper Plates. Pure copper, 7/64 inch thick, is rolled into plates or sheets for the amalgamation of gold; 2 to 4 square feet is used per ton of ore per day. New or used plates are procurable, but care should be taken in selecting old ones because they may be uneven or thin and have small holes.
Copper plates are scoured with sand, washed with a strong solution of soda ash, and then rubbed with a rag in a 1 per cent solution of sodium cyanide. The plates are then washed with water, and a mixture of fine sand, mercury, and sal ammoniac is applied with a stiff brush until the plate acquires a thin film of mercury. Half an ounce or more of mercury per square foot of plate is then squeezed through fine-woven cloth on to the plate and rubbed in with a piece of rubber or a cloth soaked in a ½ per cent solution of cyanide, and the plate is again washed with water.
A coating of silver amalgam (made by dissolving silver foil in mercury or adding a 10 per cent solution of silver nitrate to the mercury) is advantageous, according to the Equipment Company.
Best amalgamation will be obtained when:
- The ore is finely enough ground to liberate the gold.
- The pulp is dilute enough (20 per cent solids or less) to allow the metallic particles to settle to the mercury.
- The slope (of plates) is enough to prevent sand accumulation (¾ to 1½) inches for soft ore and 1½ to 2½ inches for heavy ore). The pulp should flow in a series of waves.
- The amalgam (also on plates) is pasty and bright.
- No oil or grease is allowed to come in contact with the mercury.
- The amalgam on the plates is not allowed to get thick enough to scour off.
- The gold is clean, not tarnished.
(Use care in handling cyanide, which is a deadly poison.)
If copper plates become discolored while pulp is flowing over them, the ore or mill return water may be acid. A cure for this is to add wood ash or lye, if available, or lime to the ore as it is fed to the stamps or ball-mill.

Examples of Amalgamation
If the gold in an ore is free or mostly so, there is no reason why a high recovery may not be obtained, and two examples will be given from reports of the Federal Bureau of Mines:
- At the Yellow Aster mine, Randsburg, California, gold lies in small fractures and joint-planes in granites which are mostly soft and oxidized. The gold is free and fine, and a little pyrite, arsenopyrite, and scheelite are seen sometimes. Silver occurs in the proportion of 1 ounce to 3 ounces of gold. The sorted ore is crushed by 1080-pound stamps through 30-mesh slot screens. Each battery of five heads has a chuck-plate, lip-plate, splash- plate, and a 5- by 14-foot apron-plate, with riffles at the lower end. Mercury is fed to the mortar-box and to all plates. Apron-plates are cleaned daily, and a general clean-up is made once a month. The loss of mercury averages 0.9 ounce per ton of ore milled. During a period of over 30 years, amalgamation recovered 0.167 ounce of gold per ton and left 0.0325 ounce in the tailings. This means a saving of 80 per cent. The tailings are now being cyanided in simple manner at low cost.
- At the Gold Hill mine, northeast of Boise, Idaho, the ore assays $6 to $8 per ton, and 92 per cent is saved by amalgamation. The gold is free in quartz but closely associated with a lead- bismuth mineral. The ore is crushed through 35 mesh in a ball- mill, and the first gold is caught in hydraulic traps in this grinding circuit. The pulp is next distributed to mercury-coated copper plates, which are followed by more traps, and then goes over rougher shaking tables. Middlings are ground finer and are sent
to copper plates followed by traps. This pulp then flows over slime tables, and high-grade cut is taken from them. All the material caught in the traps and that from the slime tables are ground with mercury; this and the amalgam from the plates are retorted; and the gold is melted. Consumption of mercury is 0.045 ounce per ton of ore. The copper plates save 24.2 per cent of the gold; slime tables, 22.4 per cent; hydraulic traps, 38.7 per cent; and ball-mill elevators, 14.7 per cent. The total cost of milling is 62 cents or 31 pence per ton.
Figure 131 represents the flow-sheet of a 10-stamp mill for crushing and concentrating a $10 gold ore containing some coarse gold and sulphides, as suggested by the Equipment Company. As shown, 80 per cent is recovered; but if a re-grinding mill and classifier, with more flotation cells and another table, are installed, an additional $1.75 per ton may be
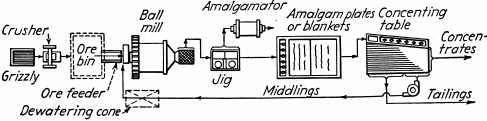
Fig. 133.—Mineral jig ahead of amalgamation. This is an inexpensive plant where gold is coarse but coated or filmed and will not amalgamate readily on plates. The jig recovers rusty gold in a high-grade concentrate for forced amalgamation treatment in an amalgamator. On these ores, blankets, corduroy, or matting usually is substituted for amalgamation plates and their concentrate is also treated in amalgamator. A 5- to 10-ton plant costs $2200 to $2500.
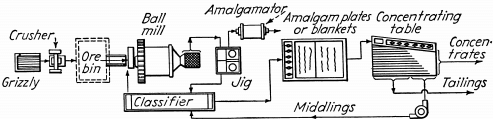
Fig. 134.—Jig in ball mill-classifier circuit. This flow-sheet with the addition of the classifier allows finer grinding and the jig used in the closed grind circuit has increased efficiency. It will give the highest recovery possible for amalgamation and gravity concentration. A 5- to 10-ton plant costs $2500 to $2750.

FIG. 135.—A retort for amalgam or mercury containing gold. This represents one of ½-gallon volume and holds 50 pounds of mercury; the retort weighs 25 pounds.
recovered. A jig could be used instead of the flotation cells shown, or the shaking table alone could follow the plates or corduroy, but perhaps the recovery would not be so high as with the cells or jig.
Figures 132, 133, and 134 are flow-sheets of amalgamation- concentration plants for a gold ore containing some sulphides as suggested by Equipment Company, Colorado.
Cleaning and Retorting Amalgam
Mercury that has collected gold on copper plates, sluices, barrel, pan, or cloth materials should first be cleaned of sand, iron, and other material. This is done by skimming them off in the riffles or in a mercury bucket, adding more mercury to make the whole liquid. The clean metal may be all retorted, or it may be squeezed through any close-woven cloth or a chamois skin, leaving a ball of amalgam which is retorted.
If pipe and tools are available on a claim, a homemade retort and condenser may be built. Otherwise, it is better to buy one from a machinery firm or assay and chemical supply house. Retorts are made in sizes of 1-pint to 1-gallon water capacity and cost several dollars. Figure 135 gives details of a retort setup.
A furnace using any type of fuel may be made of brick or of rock, or a forge may be used. The retort should stand on a brick with the fuel all around it. Before the amalgam is placed in the retort, the inside of the bowl should be washed with a thick clay or lime emulsion or lined with paper. The lid should be clamped right, using a clay or lime joint. Heat the retort gently at first, and later increase the heat greatly.
The curved pipe from the retort top may be cooled for condensing the mercury vapor by means of wetted sacks or by a regular condenser that surrounds the pipe and through which cold water circulates. The condensed mercury runs into a bucket of water. All condenser discharge pipes should have a piece of burlap tied over the end and hanging in the bucket which is collecting the mercury. The end of the pipe should never be submerged in the water which is in this bucket; only the burlap. When the mercury stops, retorting is finished. The fire by this time has been allowed to burn down.
The hot retort is lifted out of the fire, and the top removed. (At this time a little mercury vapor will rise from the bowl; so stand aside.) The retorted gold is taken out and sold locally or sent insured and with instructions to the Mint at San Francisco or Philadelphia. There is no need to melt it.
Final Cleaning of Copper Plates
Because of the need for cash or because a mine is to be abandoned, the copper plates are cleaned of all amalgam that has been absorbed. This may be done by covering them where they are with sacking or blanketing and heating them with steam from a pipe or hose. Then the amalgam can be scraped off. Or the plates may be lifted off their tables, placed on bricks or rocks built up a foot or so, coated with a solution of sal ammoniac, and heated by a wood fire under the plates. The amalgam can then be scaled off. (During this heating process some mercury vapor will be given off, so men engaged in this job should be well out of range of the vapor.
The amalgam and scale from such operations may be mixed and ground in a mortar or barrel with mercury and retorted or may be melted as is.
Sickening or Flouring and Reviving Mercury
Mercury is sickened, and perhaps floured, by all greasy materials, talcy minerals, acid water, soluble salts in ore, heavy iron pyrite, arsenopyrite, metallic oxides, mill water too cold, carbonaceous minerals as graphite in schist, and sulphides as antimony, bismuth, and copper.
It is important to keen mercury as clean and pure as possible; otherwise amalgamation will be poor and losses of mercury and gold follow. The metal may be purified by retorting it alone or with lime and fine iron. Sodium amalgam will revive sickened mercury. It is made by warming some mercury in a basin or flask and by some means dropping on to or holding under the mercury pea-size pieces of sodium. (There is some risk of burns while doing this, so one must be careful.) About 3 per cent sodium is enough to make the amalgam. It is then poured on to a flat surface to cool, broken up, and placed in a bottle under naphtha or kerosene. But as sodium amalgam is not used often, it should be made as required. If any mercury needs treatment to make it more active, stir in a few pieces of the sodium amalgam or dissolve it in some clean mercury and then add to the foul stuff. Sodium is obtainable from chemical supply firms and costs 20 cents per pound wholesale; sodium amalgam is also procurable.
While on the subject of amalgamating gold, we may suitably include something on platinum: Black sands or concentrates that contain platinum and have been re-concentrated with the iron minerals removed by magnet may be ground and analgamated in a barrel or other machines, as described.
Amalgamation is promoted by the use of zinc amalgam, copper sulphate, and sulphuric acid. After being cleaned, the amalgam is squeezed and treated in earthernware jars with dilute sulphuric acid which dissolves zinc and iron. Then the amalgam is retorted. The retorted platinum may contain 70 per cent platinum metals.
