Research on cyanidation methods brought considerable discussion on the reactions involved in the leaching and decomposition of pyrrhotite in cyanide solutions. Pyrrhotite has one loosely held sulphur atom which readily reacts with cyanide to form thiocyanate: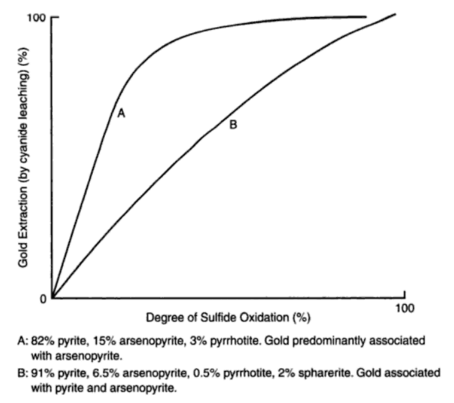
Fe5S6 + NaCN = NaCNS + 5 FeS
The ferrous sulphide oxidizes rapidly to the sulphate which reacts with cyanide to form ferrocyanide:
FeS + 2 O2 = FeSO4 FeSO4 + 6 NaCN = Na4Fe(CN)« + Na2SO4
Thus, pyrrhotite is not only a cyanicide but it also consumes oxygen in solution necessary for gold dissolution.
Pyrrhotite, if kept dry, is stable but in the presence of water and air, it breaks down rapidly forming such compounds as sulphuric acid, ferrous sulphate, basic iron sulphates, ferrous carbonates and hydrates. In this respect it is similar to pyrite and marcasite, the main difference being that the rate of decomposition of pyrrhotite is markedly greater. There is thus always the danger of appreciable decomposition taking place ‘ between the time of mining a pyrrhotite ore and treating it. The probable reactions of cyanide with ferrous compounds already in the ore were stated to be as follows:
In non alkaline solutions, ferrocyanide is formed. FeSO4 + 6 KCN = K4Fe(CN)6 + K2SO4 at the expense of much cyanide but no oxygen.
In alkaline solutions, ferrous hydroxide is precipitated and oxidized to ferric hydroxide:
FeSO4 + Ca(OH)2 = Fe(OH)2 + CaSO4
2Fe(OH)2 + O + H2O = 2 Fe(OH)3
The ferric hydroxide is insoluble and does not consume cyanide but oxygen has been used up thereby impairing the solvent efficiency of the cyanide solution for gold. Ferrous hydroxide will react with cyanide to form ferrocyanide but others suggest that in practice the oxidation to ferric hydroxide takes place first. The direct action of nonalkaline cyanide solutions on ferrous sulphides, which may be similar to their action on pyrrhotite, may be expressed as follows:
FeS +7 KCN + H2O + O =
KCNS + K4Fe(CN)6 + 2 KOH
whereas in alkaline cyanide solutions this reaction is favored:
2 FeS + 2 KCN + 3 H2O + 3 O =
2 KCNS + 2 Fe(OH)3
Reactions may take place between ferrous sulphate in the untreated ore and ferrocyanide in a cyanide solution forming a white precipitate of ferrous ferrocyanide:
2 FeSO4+ K4Fe(CN)6 =
Fe2Fe( CN )6 + 2 K2SO4
Ferrous ferrocyanide oxidizes readily in the presence of oxygen to ferric ferrocyanide or Prussian blue:
3 Fe2Fe(CN)6 + 3 O + 3 H2O = Fe4
[Fe(CN)6]2 + 2Fe(0H)3
It would thus tend to retard gold dissolution. A non alkaline solution condition would favor this reaction because prussian blue decomposes in the presence of lime to ferrocyanide and ferric hydroxide. An example was cited in the treatment of a pyritic ore. Coincident with a period of high residues, the sand on the dump showed blue coloration after exposure to the air. Discard of the “foul” solution immediately rectified matters and no blue was formed on the dumps. The foregoing reactions were quoted as typical examples to show how the various ferrous compounds formed before and during cyanide treatment tend to deplete solutions of free oxygen and so render them less effective as solvents for gold.
Some ores sometimes respond to a treatment that may he described as “preaeration in alkaline water”. In brief, the process is to add lime sufficient to maintain protective alkalinity and agitate with aeration before the addition of cyanide. The ferrous compounds are precipitated as hydrates and are oxidized to the ferric state. This may take a few hours. The completion of oxidation may be ascertained by the following test: Transfer some of the pulp to a bottle and add a small amount of solid sodium cyanide. Shake for several minutes and filter. Acidify the filtrate with hydrochloric acid and add ferric chloride. A prussian blue precipitate indicates the presence of ferrocyanide and hence that the oxidation of ferrous compounds is incomplete. Aeration is continued until the test shows no prussian blue; oxidation having been completed. Cyanide is then added to the charge and agitation and aeration continued.
According to leaching metallurgists, too alkaline a solution may directly attack sulphides. The former suggests the equation:
12 Fe5S6 + 3Ca(OH)2 =
2 CaS5 + CaS2O3 + 60 FeS + 3 H2O
and the latter:
FeS + Ca(OH)2 = Fe(OH)2 + CaS
The addition of a lead salt or a mercury salt accelerates the oxidation of soluble sulphides or polysulphides. The function of the lead or mercury salt is to precipitate the sulphide as it is formed as the insoluble metal sulphide which then is oxidized to the sulphate. The metal sulphate is redissolved by the lime or cyanide and is thus available to precipitate more soluble sulphide, The metal salt thus acts as a catalyst.
Preconditioning in alkaline solution causes the formation of a protective oxidation film on the surface of the pyrrhotite which inhibits reaction between the mineral and cyanide. He stated that solutions saturated with air at normal temperature and pressure contain approximately 8 grams of oxygen per metric ton. This amount of oxygen would be eliminated by the complete oxidation of only 8 grams of pyrite in the ore. Thus, even if a very little sulphide were oxidized, the solution would be deoxidized and no extraction of gold could take place.
Leaching researchers describe cyanidation practice in treating a given pyrrhotitic gold ore with the following average chemical composition: Au 0.42 oz./ton; Fe total 22%; Fe ferrous 20%; S total 5%; S by evolution 3.3%; CaO 6%; MgO 2.6%; As 1.5%. Pyrrhotite is the most abundant mineral in the ore, arsenopyrite is second and chalcopyrite is rare. The pyrrhotite has a chemical composition corresponding to Fe5S6 and in amount, varies from 8 to 11% of the ore. A considerable amount of ferrous iron over and above that in the pyrrhotite is present, and is thought to occur as ferrous carbonate and ferruginous calcium magnesium minerals. The ferrous iron thus associated amounts to about 10 percent of the ore. The amount of arsenopyrite varies from 0.5 to 1.5%. It is known that the arsenopyrite itself is not a serious problem in this ore, as proved in the past by mining selectively where this mineral occurred in massive form, and closely observing mill reaction. The gold occurs in very fine particles. At this mill, grinding is done in cyanide solutions and no pretreatment of any kind is practiced. The fact is accepted that pyrrhotite will decompose, and measures are utilized to prevent excessive consumption of reagents and interference with the dissolution of the gold. In the main the treatment consists of using a short period of contact, with the judicious use of litharge and careful control of the alkalinity of the circuit. The conclusions arrived at as a result of experimental work are as follows:
- Litharge (PbO) reduces reagent consumption. Working with freshly prepared solutions, it was definitely established that moderate quantities of litharge (0.50 lb/ton ore) when added to the ore at the beginning of treatment, yielded the most economical reagent consumption. The consumption of lime was decreased from 3.0 to 2.0 lb/ton of ore and the consumption of cyanide from 1.55 to 0.55 lb. NaCN/ton of ore. At the same time the thiocyanate content of the solutions decreased markedly, and the ferrocyanide content, slightly. There was also a definite drop in thiosulphate content. The author found that the presence of thiosulphate in the solution seemed to interfere with the determination of free cyanide.
- Litharge accelerates gold dissolution. — The use of litharge was found essential to produce a good tailing in the period of agitation available. Under conditions applicable to plant practice (i.e. using classifier overflow and mill solution), as low as 0.20 lb. of litharge/ton of ore was found to accelerate the dissolution of gold greatly. Within a period of 8 hours agitation, a tailing assaying between 0.010 and 0.020 oz. gold/ton was obtained when using litharge, whereas without it the residue would assay 0.060 to 0.120 oz. gold/ton. There was no advantage in increasing the litharge up to 0.80 lb/ton. When using freshly prepared cyanide solutions, litharge was not essential to produce a satisfactory tailing.
- The effectiveness of litharge is limited. When lime alkalinity of the solution was much higher than 0.020 percent CaO at the start of agitation, litharge was of no benefit. As much as 0.60 lb. of litharge/ton of ore did not counteract the effect of high lime alkalinity. The optimum lime alkalinity range was found to be 0.013% CaO at the start of agitation, falling off to 0.005% CaO at the end of 8 hours agitation. It was found necessary, however, to maintain a certain minimum lime alkalinity. A pulp charge starting with a lime alkalinity of 0.008% CaO in solution and ending with trace alkalinity yielded a very high tailing.
- Sodium hydroxide within the limits studied has no effect on gold dissolution. — In the presence of litharge it was found that solutions containing sodium hydroxide equivalent to 0.065% CaO were as good gold solvents as those in the optimum lime alkalinity range.
An EXAMPLE ore deposit is know for its gold bearing pyrite concentrate containing pyrrhotite and chalcopyrite is preaerated in a high lime solution ahead of cyanidation. Optimum results as regards gold extraction and cyanide consumption are obtained when using well aerated cyanide solutions containing at least 0.05% CaO and saturated with calcium sulphate. Laboratory results showing the cyanicidal effects of pyrrhotite under various conditions were given. In each test 50 grm. of washed, minus 325 mesh pyrrhotite and 750 ml. of cyanide solution containing 0. 05% NaCN and 0.11% CaO were used.
- In Test 1 the pulp was agitated in a 3 gallon narrow-mouthed bottle on rolls for 4 hours; this was considered moderate aeration.
- In Test 2 the pulp was agitated in a 1000 ml. graduated cylinder for 4 hours using 12 to 15 cu. ft. of air per hour; this was considered intense aeration.
- Test 3 was similar to Test 2 except that 0.135% CaSO4 was added to the cyanide solution. After 2 hours aeration 1 gram of calcium hydroxide was added to each pulp in order to maintain high alkalinity. At the end of the 4 hour period the pulps were filtered and the filtrates analyzed for free cyanide, lime, thiocyanate and ferrocvanide.
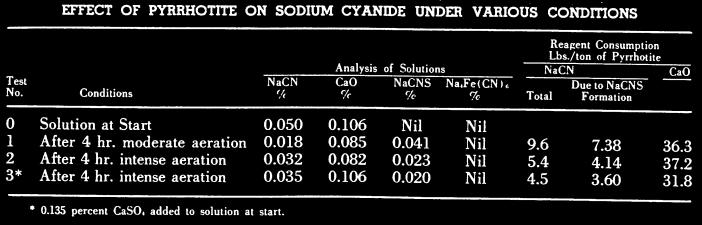
The results of the foregoing tests show that intense aeration of pyrrhotite in a strongly alkaline solution reduced the cyanide consumption by 4 to 5 lbs. of NaCN/ton of pyrrhotite. The greater part of this saving was due to less formation of thiocyanate under conditions of intense aeration. The addition of calcium sulphate to the cyanide solution was beneficial to a small extent in reducing thiocyanate formation. No ferrocyanide was found in any of the solutions.
The hypothesis was suggested that one of the initial products of the reaction between pyrrhotite and alkaline solutions is alkaline sulphide (or polysulphide). The alkaline sulphide in the presence of oxygen may decompose in two ways simultaneously. On the one hand, independently of the sodium cyanide present, by a series of reactions during which thiosulphate, sulphate and possibly other oxidized sulphur compounds are formed; for example:
2 Na2S + 2 O2 + H2O = Na2S2O3 + 2 NaOH
Na2S2O3 + 2 NaOH + 2 O2 = 2 Na2SO4 + H2O
On the other hand, sodium cyanide may enter into the reaction with the formation of thiocyanate thus:
2 Na2S + 2 NaCN + 2 H2O + O2 =
2 NaCNS + 4 NaOH
The relative proportions of alkaline sulphide decomposing by the two series of reactions may depend on the intensity of aeration. With intense aeration, the first series of reactions is favored, during which thiosulphate, having little effect on cyanide, is formed. With less oxygen present, the formation of thiocyanate is favored and cyanide consumption increases accordingly.
In the cyanidation of precious metal ores containing pyrrhotite, there is no general agreement as to the test procedure to follow and 110 hard and fast rules can he laid down. This is 110 doubt due to the complexity of the individual ores and also to the possibility that two different methods of treatment may have the same final effect on gold extraction and cyanide consumption. Some of the procedures appear to he contradictory; therefore in testing a new ore several different schemes of treatment should he investigated. The following points may he of significance:
- Preaeration in slightly alkaline solutions in the absence of cyanide. This oxidizes any soluble ferrous salts which may he present in the ore. It thus minimizes the subsequent formation of ferrocyanides when cyanide solution is added thereby reducing cyanide consumption. It also oxidizes the surfaces of sulphide minerals such as pyrrhotite, marcasite and pyrite thereby inhibiting reaction between cyanide and those surfaces.
- Preaeration in strongly alkaline solutions in the absence of cyanide. This serves the same purpose as the first procedure. However, the strongly alkaline solution might attack the surface of pyrrhotite forming first, alkaline sulphide or polysulphide which eventually decompose to thiosulphate and sulphate.
- Using preaeration solution in cyanidation. Solutions high in thiosulphate and sulphate content inhibit the reaction between pyrrhotite and cyanide thereby cutting down the oxygen demand of that mineral. More oxygen is, therefore, available for the dissolution of gold and less cyanide is consumed.
The use of solutions containing thiosulphates, sulphates, etc., simplifies low pH control due to the buffering action of such salts. Thiosulphate at temperatures below 30 C. has no appreciable effect on cyanide in solution. - Discarding preaeration solution. Such solutions, particularly if preaeration has been conducted at low pH, might contain ferrous salts which, if used in cyanidation, would react with cyanide forming ferrocyanide. Discarding such solutions before cyaniding would save cyanide.
- Alkalinity during cyanidation. A low pH, between 9 and 10, during cyanidation favors rapid dissolution and maximum extraction of gold. This, however, leads to the formation of ferrocyanide and possibly hydrocyanic acid; the cyanide consumption may be high accordingly.
When the gold in a pyrrhotitic ore is very fine, it may not be necessary to use cyanide solutions having such low pH values in order to obtain rapid and maximum dissolution of gold. Loss of cyanide as ferrocyanide and hydrocyanic acid will thus be avoided. The main loss of cyanide in this case will he due to thiocyanate formation. - Aeration during cyanidation. With moderate aeration in strongly alkaline cyanide solutions, the loss of cyanide may be appreciable when treating ores containing pyrrhotite. Practically all of this is due to thiocyanate formation. With intense aeration under the same conditions the loss of cyanide as thiocyanate may he reduced by half.
- Use of lead salts. Lead salts, such as litharge, lead nitrate, etc., when added to cyanide solutions of low lime alkalinity, accelerate the dissolution of gold and reduce reagent consumption, particularly cyanide lost as thiocyanate. Pre-aeration, and intense aeration during cyanidation may not be required when lead salts are used in conjunction with low lime alkalinity.
Lead salts do not overcome the detrimental effect of cyaniding in high lime solutions. - The use of other metallic salts. The addition of zinc salts, and particularly mercury salts may improve cyanidation results; their function is probably analogous to that of lead salts.
