Table of Contents
The use of aluminium as a precipitant for gold out of cyanide solutions was patented by Moldenhauer but he does not appear to have made any practical use of the process. H. F. Julian experimented with it, in the same year, but soon abandoned the idea. Kirkpatrick introduced it at the Deloro Smelter in Canada, applying the metal for the first time in the form of a powder, and achieved a complete success. He later installed it at the O’Brien Mine in Cobalt.
When making tests on the cyaniding of the Nipissing low- grade ore it was found that the arsenic and antimony dissolved out of the ore by the cyanide formed a harmful combination with the zinc derived from zinc precipitation causing a serious loss of dissolving power in the stock solutions. Consequently an alternative method of precipitation was sought for, and that in use at the O’Brien mine naturally suggested itself. The experiments were so successful that the process was introduced in the new mill at its start and the results fully justified the choice, the stock solution at the end of twelve months being more active for dissolving silver than freshly made up solution.
The fact that aluminium does not replace the precious metals in the cyanogen compound renders necessary the presence in the solution of caustic soda. There are two possible equations to represent the reaction incident to precipitation.
The first is suggested by Moldenhauer:
6NaAg(CN)2 + 6NaOH + 2Al = 6Ag + 12NaCN + 2Al(OH)3, the aluminium hydroxide at once dissolving in the excess of caustic to form sodium aluminate, 2Al(OH)3 + 2NaOH = Na2Al2O4 + 4H2O
This would indicate that one part of aluminium should theoretically precipitate twelve times its weight of silver, but in practice the amount is about three times its weight only, so the following may more nearly represent the actual reaction,
2NaAg(CN)2 + 4NaOH + 2Al = 4NaCN + 2Ag + Na2Al2O4 + 4H
The method of working is somewhat similar to that for zinc dust but the action is slower in practice, and necessitates a receptacle in which to retain the emulsion under agitation until the action is complete. The dust also is not nearly so easily wetted as zinc dust and a special form of agitator is used to assist in effecting this. The reason why the action is so slow is probably a mechanical one, because if an excess of dust be used in a strongly caustic solution the reaction is rapid, about two minutes only being required for total precipitation, but in practice the amount of precipitant used has to be cut down to the lowest possible point for the sake of economy and consequently a proportionately longer time is needed to bring each molecule of the cyanogen compound in contact with the metallic surface of an aluminium particle. It follows from this, at least in the case of solutions of the average low metallic content, that the quantity of precipitant necessary depends more upon the volume of solution to be precipitated than upon its assay value, and therefore it is more economical to concentrate the precious metal into as small a bulk of solution as possible for precipitation rather than to precipitate large volumes of low grade solution.
One point in the chemistry of the process is vital to its practical success and that is that at the time of precipitation lime must be absent, as it decomposes the sodium aluminate to form insoluble calcium aluminate,
(Na2Al2O4 + Ca(OH)2 = CaAl2O4 + 2NaOH),
which deposits in the press with the precipitate, making a large bulk of low-grade product which it is almost impossible to melt into bullion. At the Nipissing this condition of absence of lime does not present any difficulty because the slime settles well for the final decantation in the cyanide liquor with only the merest trace of lime, but difficulties are likely to arise with other ores from this cause and to meet such cases the writer devised the modified process described later.
The reaction between the lime and the sodium aluminate is turned to useful account after precipitation in preventing an undue accumulation of the sodium aluminate in the stock solution, because when the barren solution next comes in contact with the lime added in the mill it is broken up, the aluminium being precipitated as the insoluble calcium compound with liberation of caustic soda.
There is usually a tendency to a formation of soluble sulphides as the result of precipitation by this process. It has been suggested that this is caused by a decomposition of the soluble sulphocyanates present, but laboratory tests made in weak solutions of KCNS (0.05% to 0.15%) have failed to show any signs of such a reaction. Sodium thiosulphate is easily decomposed by aluminium dust with formation of sodium sulphide, so where soluble sulphides are found after precipitation they are probably due to the presence in the stock solution of thiosulphates.
At the Nipissing mill the formation of soluble sulphides was considerable and a few drops of lead acetate added to a little of the effluent from the press in a beaker would turn it a dead black. As the addition of lead salts to neutralize this sulphide seemed to have a prejudicial effect on the subsequent extraction of silver from the ore, the expedient was adopted of installing an air lift in the barren sump, and a few hours agitation in this way served to remove every trace of soluble sulphide, rendering the solution perfectly efficient in dissolving power when next brought in contact with the ore pulp. At the Butters Divisadero mill only traces of soluble sulphide were ever found in the press effluent and none at all in the barren sump, and it was not found necessary to take any precautions to counteract their effect.
How the method of precipitating gold with aluminum works
The apparatus necessary is similar to that in use. for zinc dust with the addition of two small agitator tanks to afford the additional time of contact of the solution with the precipitant. Owing to the difficulty of wetting the dust a special form of agitator is used. The tanks for treating 600 tons of solution a day may be 6 ft. in diameter and 4 ft. deep. In each is a central shaft resting on a bearing on the tank bottom and rotated at from 40 to 60 revolutions per minute by means of a horizontal shaft with crown and pinion or by a quarter-turn belt. Bolted vertically onto the central shaft on opposite sides of it are two boards 10 inches wide and 1 inch thick, extending from the bottom to within 12 inches of the top of the tank. This device imparts a peculiar boiling motion to the contents of the tank quite unlike that produced by the ordinary long paddles at the bottom of a slime agitator, and the fact that the boards extend upward so near to the top of the tank serves to break the smoothness of the surface of the liquid, breaking up at the same time the film of aluminium dust which would otherwise have a tendency to float and accumulate there.
The two tanks are connected in such a way that the emulsion in the first is maintained at a constant level and overflows into the second whose function is chiefly that of a sump from which to operate the pump.
The latter is at the Divisadero mill fitted with a bypass on the delivery, leading back into the sump, and the flow through the by-pass is regulated by an automatic float valve, the object being to maintain a constant head of solution over the suction and prevent the entry of air into the pump. A better plan than this, however, is to arrange the float valve to control the main flow of solution from the pregnant storage into the precipitating tanks as this avoids the additional aeration of the solution caused by the entry of the stream flowing from the by-pass.
The following data refer to an installation capable of precipitating from 500 to 600 tons of solution a day.
The solution after leaving the clarifying filter passes down through a covered launder to the precipitation tanks. In this launder is added the aluminium dust, through a worm-feeder whose speed is adjusted by means of a pair of cone-pulleys. When it nears the precipitation tanks the continuity of the launder is broken twice, allowing the solution a clear drop of 6 inches each time, to assist in wetting the dust.
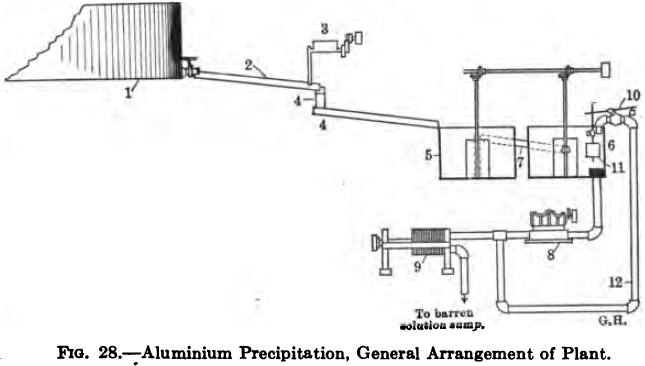
Referring to Fig. 28 (1) is the clarifying filter, (2) launder, (3) the aluminium-dust feeder, (4) interruptions in the flow of solution, (5) and (6) the precipitation tanks. (7) is a 6-in. pipe connecting the two tanks; it extends almost to the bottom of (5), and after passing through the side near the top it slopes down somewhat and discharges into (6). It will be seen from this that the solution in tank (5) is maintained at a constant level, while the level of that in (6), though normally constant, can be varied at will, by raising or lowering the float (11) on the rod operating the automatic cut-off valve (10). (8) is a triplex 7 X 7 in. plunger pump running at 29 r.p.m. and drawing through a 5-in. pipe from tank (6) discharging into the filter-press (9). (12) is a 2½-in. by-pass line in the delivery of the pump, and its discharge is regulated by the automatic float-valve (10). Should the supply of solution from the clarifier be slackened off the level of solution in (6) would tend to fall, thus opening valve (10) and allowing the surplus solution passing the pump to return to the tank, in this way maintaining a constant level.
The importance of this lies in the necessity of keeping the pump-suction water-sealed and preventing the entry of air. The operation of precipitation being a reducing one, the presence of air tends to retard it; moreover the silver precipitate formed by aluminium dust is so sensitive to re-solution influences that air in juxtaposition with cyanide solution will often cause the metal to re-dissolve in the filter-press itself.
There is also on the pump-delivery a pressure-gauge, and a relief-valve (not shown in the diagram) set at 80 lb., and discharging back into tank (6).
Hamilton’s Process of Gold Precipitation
At the Nipissing mill the small amount of lime added in the battery is almost completely eliminated from the ore and solution by the time the latter is ready for precipitation, consequently no trouble is experienced by the deposition of calcium aluminate in the press; and no caustic soda is added before precipitation because more than sufficient for this purpose passes into the cyanide plant from the preliminary desulphurizing process. Neither of these conditions, however, is likely to be encountered in the majority of mills where the process might be introduced and to meet circumstances where the Nipissing routine is inapplicable the writer devised the following process, which was worked out at Butters Divisadero Mine with the assistance of P. H. Crawford, the resident metallurgist.
It consists in treating the solution before precipitation with soda ash, thus throwing out the lime as carbonate and substituting caustic soda therefor:
Ca(OH)2 + Na2CO3 = CaCO3 + 2NaOH
CaSO4 + Na2CO3 = CaCO3 + Na2SO4
It allows the use of all the lime necessary for neutralizing and settlement, it yields a lime-free solution for precipitation, and incidentally manufactures the caustic soda necessary for that operation, in accordance with the formula, given above.
The method of operation is as follows: The pregnant solution is pumped to a tank of 100 tons capacity, above the plant, fitted with a mechanical stirring-gear, and it is here that the soda ash is added. In order to find out exactly how much of the latter is needed, a sample test is employed. A standard solution of the soda ash is prepared by dissolving 6.25 grams in distilled water and making up to 1 litre, and is such that if 50 cc of pregnant solution be taken for the test, each cubic centimeter of soda ash solution will be equivalent to ¼ lb. of the dry salt per ton of cyanide solution. A portion of the solution to be tested is filtered, and 50 cc measured and placed in an Erlenmeyer flask or small beaker and heated over a flame until almost too hot to hold in the fingers. A measured quantity, say, 2 cc of the soda ash solution is then drawn from a burette and agitated for a few minutes to insure complete precipitation. The contents of the flask are filtered, and the filtrate divided into two parts: to the first part is added a few drops of potassium oxalate or sodium carbonate solution, and if any turbidity results it indicates an insufficiency of soda ash. In that event a fresh portion of the original solution is taken and the test repeated, increasing the soda ash to 2.5 or 3 cc, filtering, and testing as before. This operation is repeated, if necessary, until no turbidity is produced on adding oxalate. When a test is secured in which no lime is indicated, the second portion of the filtrate is tried for excess of soda ash, by adding a few drops of calcium chloride solution. If no turbidity is produced, the required quantity has been found, and the number of cubic centimeters of soda ash solution multiplied by 0.25 and by the number of tons of solution in the tank gives the amount of soda in pounds to be added to the charge. Should the addition of calcium chloride, mentioned above, indicate that there is an excess of soda present, the original test may be repeated, using 0.5 cc less of the standard soda solution.
A slight excess of soda ash seems to be unimportant, but it is essential that no lime should be indicated.
It usually takes from 10 to 15 minutes to ascertain the amount of ash needed for a charge, and When this has been done the required quantity is placed in a screened drum hanging under the inflow pipe, and the pump and agitator started. By the time the tank is filled to the required tonnage mark the reaction is complete and the charge is allowed to settle, the clear solution being then decanted to a sand-filter clarifier, whence it passes to the precipitation house. The turbid remainder is afterward stirred and run through a valve in the bottom into a smaller tank below fitted with a filter-bottom connected to a vacuum-pump; here the carbonate of lime settles and allows a further decantation of clear solution to the sand-filter. The result of several days’ operations is allowed to accumulate in this filter-tank, and finally the vacuum is turned on and the precipitated lime filtered to a firm cake. After a water-wash, to remove pregnant solution, the deposit is shoveled into empty cyanide tins and allowed to dry in the air for a few days, and then charged back into the lime-kiln to be re-burnt and used over again. If desired the deposit of carbonate of lime may be allowed to remain in the soda tank for several days, and then after close decantation allowed to run out to the residue pulp filter storage.
The precipitate obtained by the use of aluminium dust is bulky and porous; it has a rich brown color, and usually melts readily with a minimum of flux though in some cases it appears to be somewhat more refractory than zinc precipitate. On an average it contains about 85% of the precious metals, and when melted yields bullion 980 fine.
As far as has been ascertained the process is not suitable for solutions containing only gold though the gold contained in solutions assaying a couple of ounces or more of silver per ton is almost completely precipitated. Copper is only precipitated to a slight extent even in the presence of a Considerable amount of silver.
Advantages of Aluminium as a Gold Precipitant
The use of the process as a substitute for zinc precipitation when treating silver ores containing antimony and arsenic will usually result in an increased extraction, both of gold and silver, which in some cases has been as much as 10% of the original assay value, but apart from this its chief advantage will be in the saving of cyanide in the treatment of silver ores. The equation already given shows that the whole of the cyanogen combined with silver is regenerated in the form of alkaline cyanide, and this theoretical increase in cyanide strength is borne out by titrating the solution before and after precipitation. At the Butters Divisadero Mill, however, in the course of a comparative run between zinc and aluminium, taking three representative months on each process, the reduction in cyanide consumption actually shown during the period covered by aluminium precipitation, was more than double the amount calculated as having been in combination with silver, and the balance of the saving was probably due to the elimination of a source of loss mentioned under the head of zinc precipitation, that is, the direct combination of cyanide with zinc.
There is one drawback to the process that may be in evidence where filters are used for handling the pulp direct from the treatment tanks. The filter cake before washing will obviously contain solution high in lime and when the barren solution containing sodium aluminate strikes this soluble lime an insoluble precipitate of calcium aluminate is formed, as already noted. It happens however that the reaction is not instantaneous and therefore continues after the solution has reached the canvasses resulting in a deposition of calcium aluminate in the fiber and reducing the porosity and consequently the filtration rate very materially. Moreover this deposit does not readily dissolve in weak muriatic acid so that the consequences in loss of filtration efficiency may be serious. This was not in evidence at the Divisadero mill on account of the use there of the special leaf with replaceable filter medium already described (page 111). In ordinary cases, however, if the trouble is serious it could no doubt be remedied by adding sifted lime powder or milk of lime to the barren solution sumps which for this purpose would be fitted with some form of agitator. By this means the reaction between the lime and the sodium aluminate would take place before the solution entered the filter cake, thus rendering the solution harmless in this respect. It would probably not be necessary to settle or filter out the suspended matter from the barren solution before sending it to the filter as this would merely deposit in a thin film on the outside surface of the filter cakes and would thus continually eliminate itself. In cases where the Dorr counter current system of washing is in use the trouble would not appear at all even when filtration is resorted to for dewatering the product of the final thickener, because the reaction between the lime and the sodium aluminate would take place in the thickener and be complete before the pulp reached the filter.
As to relative costs of the two processes, the figures ascertained in the above-mentioned test were, zinc $0.076, aluminium, $0.071, soda ash, $0.036, showing that the aluminium process cost $0.031 per ton more than zinc but resulted in a saving of $0.18 per ton in cyanide, giving a net profit of about 15¢ per ton of ore, and this on an ore containing only 7 ounces of silver per ton.
