Table of Contents
When dry chlorine gas is made to act in the cold upon finely-divided gold, it converts the latter with evolution of heat into auro-auric chloride, Au2Cl4, a hard, dark-red, hygroscopic salt. Moisture splits this salt into aurous and auric chloride, Au2Cl4 = AuCl + AuCl3 ; treatment with water converts it into auric chloride and gold, 3 Au2Cl4 = 4 AuCl3 + Au2. Aurous chloride, when stirred with water, undergoes a similar decomposition, 3 AuCl = AuCl3 + Au2. These decompositions of auro-auric chloride and of aurous chloride furnish the explanation for the practice of moistening an ore before it is treated by the Plattner chlorination-process; practical experience having shown that gaseous chlorine gave an unsatisfactory extraction with dry ore.
According to Rose, fine gold is acted upon more slowly by chlorine than gold containing some base metal, e.g., copper. He also says that small quantities of silver increase the rate of solution, but adds that the coating of silver chloride formed, checks, and finally stops, further action, if the percentage of silver be increased beyond a certain undetermined amount.
Bromine acts upon finely-divided gold in a manner similar to chlorine; the auro-auric bromide, Au2Br4, however, is not hygroscopic. Upon treatment with water it is decomposed as is the corresponding chlorine salt. It is generally believed that bromine does not act as energetically upon gold as does chlorine. This seems to be borne out by the results in the leaching plant of the Black Hills Milling and Smelting Co., Rapid City, S. D)., where barrel-chlorination and bromination were compared in the laboratory and in the mill, and chlorine was found to make poorer tailings than bromine. Rose, however, in experimenting upon the rate of solution of gold by chlorine and bromine, found that bromine dissolved gold more rapidly than chlorine, and that the action of both was quicker at from 50° to 60° C. than at ordinary temperature.
Percy describes two striking lecture-experiments which show how readily silver leaf is converted into silver chloride or bromide by the action of gaseous chlorine or chlorine water, or by bromine vapor or bromine water. A large bottle is filled loosely with silver leaf; upon introducing chlorine or bromine the leaf is entirely converted in a short time into white chloride or yellowish bromide. As to the effect of silver upon chlorination of gold, the only figures published are those of Dietzsch, “Wagemann and Coignet. Dietzsch and Wagemann say that the gold must be at least 0.917 fine, if a satisfactory extraction is to be obtained, and Coignet asserts that gold with from 10 to 12 per cent, of silver is more readily dissolved than pure gold, but that gold with from 40 to 50 per cent, of silver cannot be successfully treated by chlorine with a view of dissolving the gold.
In regard to the effect of silver on bromination of gold, no data appear to have been published.
In chlorinating gold with gaseous chlorine in a stationary vat, the gold should be more effectively protected by the silver chloride formed than when chlorine-water or bromine-water is used in a revolving barrel, as the coating of silver chloride or bromide formed will be removed more or less by the abrasive action of the charge upon the metallic particles.
The aim of the present research was to find the dissolving power of chlorine-water and of bromine-water upon gold and upon a series of alloys of gold and silver, the operations being carried out in revolving vessels.
The Ore-Charge:
The ore used in the experiments was made up of quartzite and gold, resp. gold-silver alloy. The quartzite was crushed to pass a 40-mesh sieve and freed from particles of iron from the crushing-machinery by means of a magnet, and by boiling with acid. The alloys were prepared from chemically pure gold and silver. The metals were rolled into strips, the desired quantities then weighed out to 0.01 mg. and alloyed by fusing on charcoal before the blow-pipe. The resulting globules were hammered to thin disks and each converted into a fine powder by holding in pincers and rubbing the edge on a fine file, using a very slight pressure. Any particles appearing to be coarse under a magnifying glass were put aside. The quantities used were too small to allow the use of a limiting sieve; the size of particles is therefore not definitely given. A charge was made up of 5 assay-tons of 40-mesh quartzite and 10 mg. of metal, making thus an ore assaying 2 oz. per ton. The composition of the series of alloys tested was—
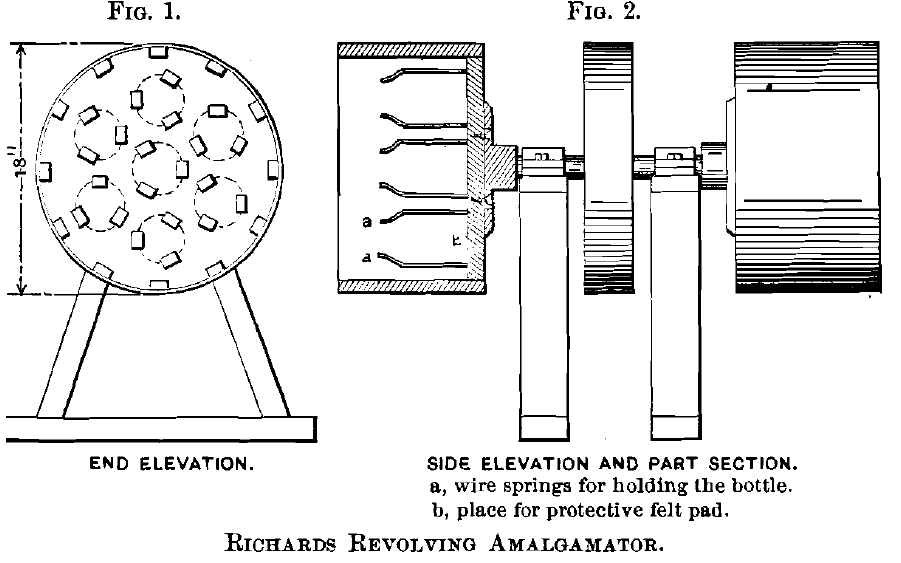
The Apparatus:
The dissolving-tests were made in pint fruit-jars of glass, 3 in. in diameter and 6 in. high, the covers being held in place by screw-clamps, and the joints made tight by rubber gaskets. The jars were rotated in the Richards revolving apparatus shown in Figs. 1 and 2. This machine consists of a horizontal shaft with pulley, having at either end a cylindrical box holding 7 jars. Each box has a wooden bottom, 12 staves connected by a stout wire, and sides of 2.5-lb. lead. Ajar is held loosely in a horizontal position by three sheet-steel springs, a, screwed to the wooden bottom. A circular felt pad, b, tacked down between the three springs, protects the bottle against jarring. The barrel makes 4.3 rev. per minute.
The Solvent:
In order to obtain a satisfactory extraction of gold by chlorination or bromination, it is essential to have an excess of reagent over that taken up by the ore. In working on a large scale, it has been found that the consumption of chemicals per ton of ore is the larger, the smaller the charge. Bringing this down to a laboratory-scale, the proportions necessary will be much greater than those found in practice. Considering further the manner of preparing the finely-divided gold or gold-silver, which was coarser than that found in ores subjected to leaching, the excess of solvent in the present case has to be larger than that required by a natural ore in a laboratory-test.
In comparing chlorine and bromine as solvents, it will be necessary to have equivalent quantities. Guided by experiences in regular laboratory practice, the quantities of bromine settled upon, using 150 c.c. of water and 5 A. T. of ore, were 3.0, 2.0, 1.5, 1.0, 0.8, 0.7 and 0.5 c.c. According to Roozeboom, a saturated solution of bromine in water contains at 0° C., 4.5 per cent, of bromine; at 3°, 3.8 per cent.; and at 10°, 3.33 per cent. The solubility decreases slowly with the temperature.
Dancer gives for 15° C., 3.226 per cent, of bromine, and for 30°, 3.126 per cent. With 1 c.c. of bromine weighing 2.99 g., the equivalent of chlorine will be 1.32 g., according to the ratio—Br : Cl =79.96 : 35.45 = 2.99 : x. The chlorine in the experiments was obtained by the action of sulphuric acid upon bleaching-powder. The available chlorine in this was determined by adding to a semi-solution of a weighed quantity of bleaching-powder an excess of a standardized solution of arsenious oxide, titrating back with a standard solution of iodine and calculating the available chlorine from the oxidized arsenious oxide. The bleaching-powder contained 20.78 per cent, of available chlorine, hence the weight of the desired amount of chlorine, in grams, has to be multiplied by 4.81 in order to obtain the necessary quantity of bleaching-powder. Multiplying by 5 gives a slight excess. For each gram of bleaching-powder used, 1.5 g. of concentrated sulphuric acid (sp. gr., 1.84) were added. The solubility of chlorine in water is limited. According to Gay-Lussac:
Table.1 Solubility of Chlorine in Water.
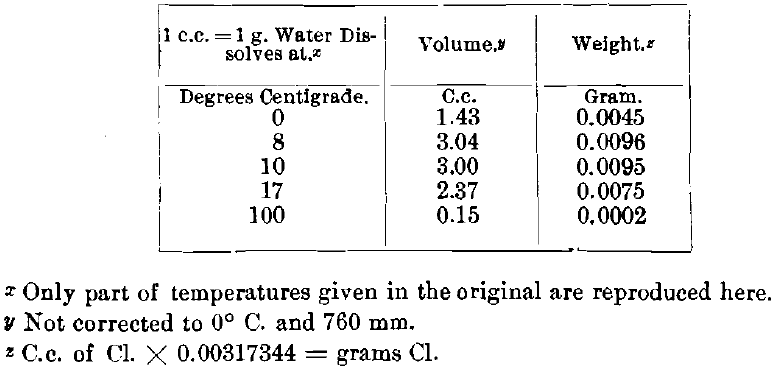
Mode of Operating:
In making up a charge, the gold-or alloy-powder was well mixed with the 40-mesh quartzite and charged into the flask. In brominating, 150 c.c. of water was added and then the required cubic centimeters of bromine dropped from a graduated pipette keeping the tip under water. In chlorinating, the bleaching-powder was mixed with the ore, the mixture filled into the flask, 150 c.c. of water added, and lastly the sulphuric acid. Although there is some danger of loss of chlorine in this method of charging, an account of the gas being sometimes evolved before the lid can be secured down firmly, it is to be preferred to charging the bleaching-powder in bulk and then covering it with the ore, as the powder is not so liable to form lumps coated with calcium sulphate, which prevents further action of the sulphuric acid and thus gives less chlorine than called for by the test. The time of rotation was uniformly 5.5 hr., the round box making 4.3 rev. per minute. When the time had elapsed, the content of a jar was transferred to a 7-in. filter and washed for from 2 to 3 hr. with from 800 to 1,000 c.c. of water, ferrous sulphate serving as reagent to test for gold; the residue and filter were placed on a roasting-dish at the mouth of a muffle, dried, the filter burned, the whole passed through a sieve to break up all lumps, then charged with the necessary fluxes into a Battersea crucible, mark K, and fused. The charge, comprising ore, 5 A. T.; sodium bicarbonate, 150 g.; litharge, 300 g.; argols, 10 g.; and the salt cover; gave in 1.5 hr. a lead button weighing about 125 g., which had to be reduced by scorification to about 25 g. before the cupellation. This was done in a 3.5-in. scorifier necessitating one pouring-off of slag. The alloy-buttons were parted in the usual way. after adding the required quantity of silver, and fusing before the blow-pipe. The conditions of assay were kept as uniform as possible, in order that any losses by slagging, volatilization and cupel-absorption might be the same, and the results correct in relation to one another.
Results:
The results obtained are given in Table II. for chlorination, and Table III. for bromination, and graphically represented in Figs. 3 and 4 respectively. The tables require no further explanation. In Fig. 3 the constant is the quantity of active reagent, the variables are the percentage of extraction drawn as ordinate and the ratios of gold and silver drawn as abscissa. In Fig. 4, which was plotted to bring out in a more striking way the dissolving-effects of chlorine and bromine, the variables are quantity of reagent drawn as ordinate and the percentage of extraction drawn as abscissa, while the composition of
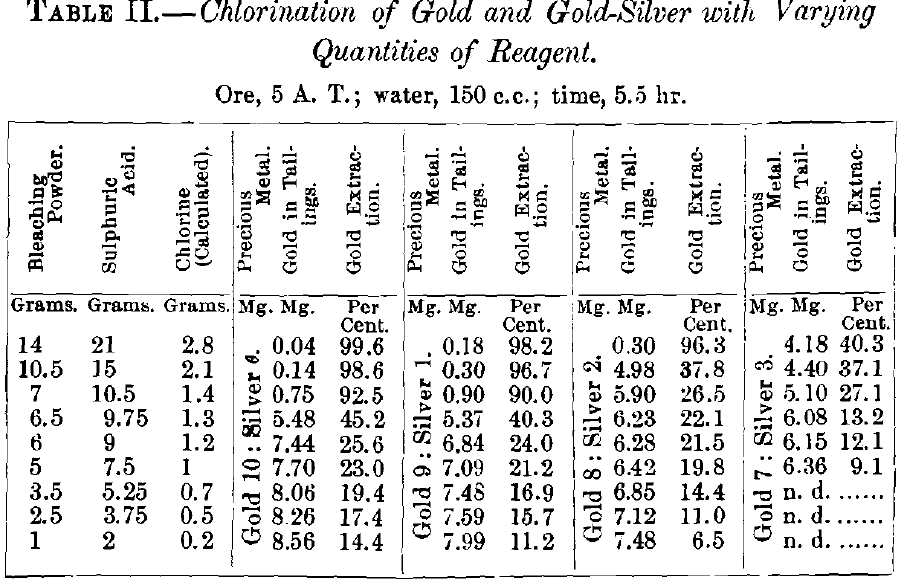
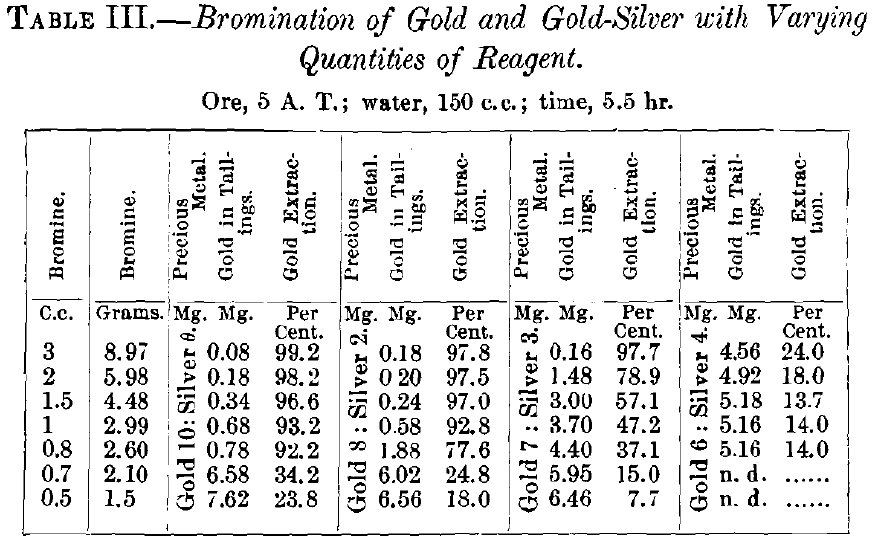
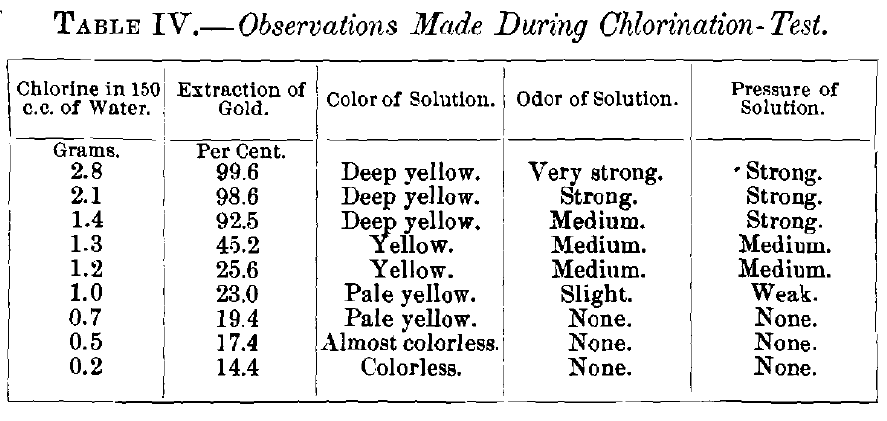
the alloy is the constant. In analyzing the results of chlorination in Fig. 3, it is seen that with pure gold the solutions containing 2.8,2.1 and 1.4 g. of chlorine give good extractions, i.e., 99.6, 98.6 and 92.5 per cent.; with 1.3 g. chlorine there is a sudden fall to 45.2 per cent., and with 1.2 g. a second decided lowering of extraction to 25.6 per cent.; from now on the yield
decreases with the diminishing quantity of chlorine present in more regular way. Observations made with the flasks during the tests are recorded in Table IV.
Gay-Lussac (see Table I.) has shown that under atmospheric pressure and a temperature of 17° C. 1 c.c. of water can dissolve only 0.0075 g. of chlorine. In order to obtain the super- saturation required for a good extraction, it is necessary to have a “ strong ” pressure in the revolving-jar. When the pressure falls to “medium” there is a decided diminution in the yield of gold.
This agrees with the experiences made in practice where it has been found that pressure of free chlorine is absolutely necessary for a satisfactory extraction, i.e., one exceeding 90 per cent. As the solution of chlorine in water, according to the law of Henry, increases with the pressure, one might be led to believe that very high pressures of free chlorine would increase the extraction of the gold, but this has been found not to be the case, as, e.g., at Deloro, Canada, no better results were obtained with 10- than with 60-lb. pressure. The essential point was to have, at first, sufficient pressure of chlorine (10 to 15 lb.) that at the end of the operation (after 2 hr.) there might be some left (3 lb. and less) to maintain the supersaturation of the solvent. The experimental data and results from large-scale work show that a satisfactory extraction of gold in barrel-chlorination can be obtained only by having supersaturated solution of chlorine in water; this requires a certain pressure, varying with the character of the ore, and has to be determined for every case.
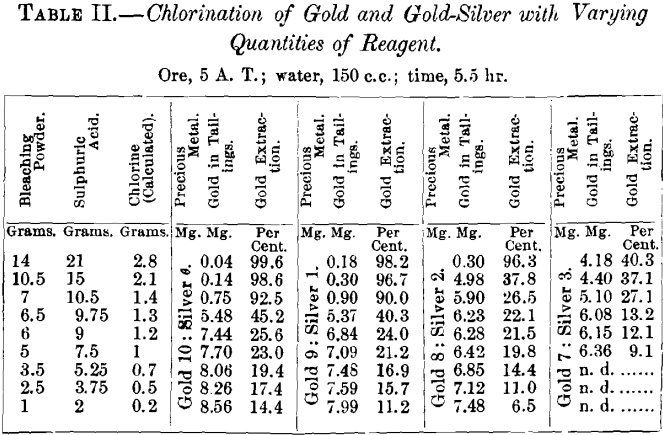
In chlorinating the alloy of 9 parts gold with 1 part silver, the extraction with 2.8 g. of chlorine falls 1.4 per cent.; with
2.1 g., 2.1; with 1.4 g., 2.5; and with 1.3 g., 4.9 per cent.; the decrease in yield growing with the diminishing grams of chlorine. With smaller quantities of chlorine, from 1.2 to 0.2 g., there is no such regularity.
With the alloy of gold 8 parts and silver 2 parts, the super-saturated solution with 2.8 g. of chlorine shows only a small decrease of 0.7 per cent, in the gold dissolved. The solution with 2.1 g. of chlorine has its dissolving power diminished by
20.1 per cent., that with 1.4 g. of chlorine by 52.2 per cent.; that with 1.3 g. of chlorine falls off less, viz., 18.2 per cent.; it loses its previously isolated position and unites with the extremely low extractions of solutions with from 1.2 to 0.2 grams.
With the alloy of gold 7 parts and silver 3 parts, the solution with 2.8 g. of chlorine succumbs to the influence of silver, dissolving only 40.3 per cent, of the gold.
The data show that a supersaturated solution of chlorine in water acts more strongly than one that is merely saturated, that a supersaturated solution can extract a satisfactory percentage of gold from a gold-silver alloy containing as much as 20 per cent, of silver, and that with the decrease of chlorine below a certain amount (2.1 g.) and the increase of silver above 10 per cent., the extraction of gold falls off quickly.
The results of bromination in Fig. 3 show that bromine also is an efficient solvent for gold, giving extractions of 99.8, 98.2,
96.6, 93.2 and 92.2 per cent, with solutions of 8.97, 5.98, 4.48, 2.99 and 2.60 g. of bromine in 150 c.c. of water; when the bromine present sinks to 2.1 g. the yield in gold falls quickly to 34.2 per cent., and then diminishes more gradually.
Observations made with the flasks during the tests are recorded in Table V.
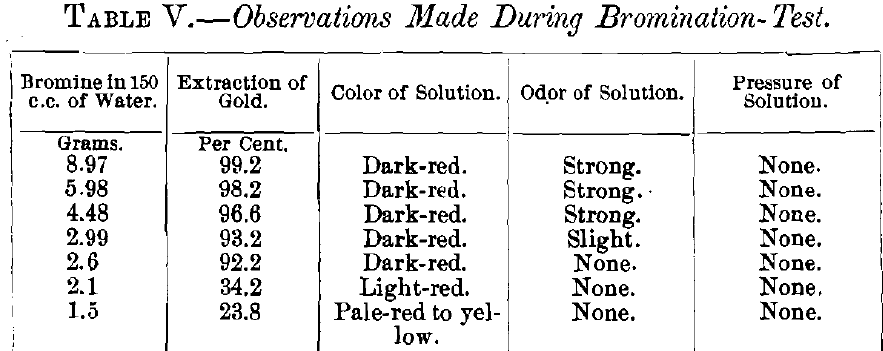
The experiments of Dancer show that at 15° C. (ordinary temperature), saturated bromine water contains 3.22 per cent, of bromine; a saturated solution of 150 c.c. of water contains 4.839 g. of bromine. The tests show that a solution with as little as 2.6 g. of bromine dissolves more than 90 per cent, of the gold when silver is absent.
The presence of 20 per cent, of silver in the gold does not make more than 1 per cent, difference in the extraction in the first four tests with 8.97, 5.98, 4.48 and 2.99 g. of bromine; the fall begins with 2.6 g. of bromine which dissolve 77.6 per cent, of gold, while in the absence of silver the same amount of bromine gives an extraction of 92.2 per cent.
With an alloy of 7 parts gold and 3 parts silver, only the solution with 8.97 g. of bromine holds its own, giving a yield of 97.7 per cent.; the other solutions show diminishing extractions corresponding with the smaller amounts of bromine present.
With an alloy of 6 parts of gold and 4 parts of silver, the stronger solutions are similar to the weaker ones in their low percentages of extraction.
The data show that 150 c.c. of water containing only 1.93 g. of bromine give a satisfactory extraction (92.8 per cent.) of gold from an alloy of gold with as much as 20 per cent, of silver, and that the extraction-figure is raised only 4.7 per cent, by doubling the amount of bromine.
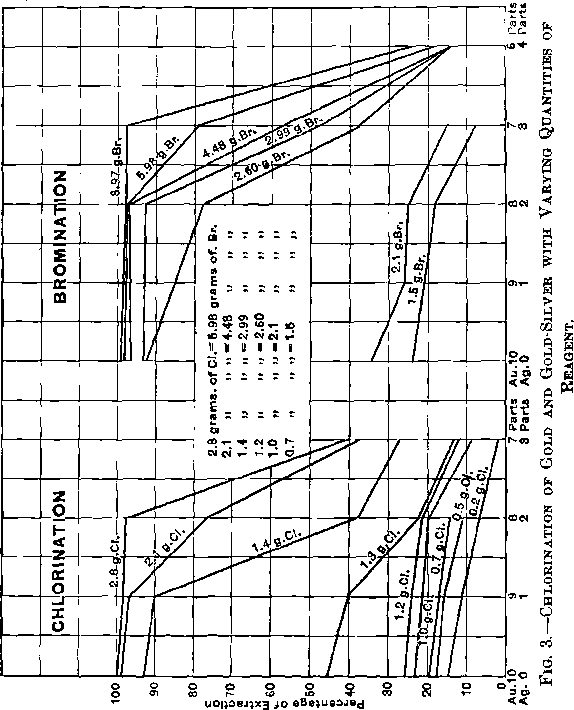
The comparison of the dissolving-effects of chlorine and bromine upon pure gold and upon gold alloyed with different amounts of silver can be seen in Fig. 3, but is more clearly illustrated in Fig. 4.
Comparing curve I (chlorine and pure gold) with curve II (bromine and pure gold), it is seen that with concentrated solutions (2.8 g. of Cl equivalent to 6.0 g. of Br), chlorine is a slightly stronger solvent than bromine; as the solutions become less concentrated the dissolving power of chlorine falls more quickly than that of bromine, until at 1.58 g. chlorine (equivalent to 3.4 g. of bromine) the power of the two is the same. With further dilution to 1.20 g. of chlorine, bromine is a much better solvent than chlorine; when the dilution is carried very far, the percentages of extraction by the two solvents come again nearer together, but bromine always remains the more efficient of the two.
Curve III shows that the extraction of gold from an alloy of gold 9 parts and silver 1 part by means of chlorine is but little affected by the presence of 10 per cent, of silver; that this is still less the case with bromine can be inferred from the bromination curves in Fig. 3, where pure gold and gold with 20 per cent, of silver are connected by straight lines.
Coming to the alloy of 8 parts of gold and 2 parts of silver, the harm done by the silver on the extraction with chlorine (curve IV) is marked, while with bromine it has very little effect. While with 2.8 g. of chlorine the extraction of gold is diminished only 2.1 per cent., it falls quickly and regularly with dilution of the solvent to 22.1 per cent, until 1.3 g. has been reached, and then falls suddenly to an insignificant figure. With bromine, dilution from 6 to 3 g. hardly affects the result, and a further addition of water only very little. Thus bromine again is the better solvent in the presence of silver.
Curves VI and VII, representing the treatment of an alloy of 7 parts of gold and 3 parts of silver, with chlorine and bromine, show how seriously the extraction of gold is affected by the presence of such large proportions of silver, but the extractions of bromine are always better than those of the equivalent quantities of chlorine.
Curve VIII, representing the alloy of gold 6, and silver 4, finally shows that even bromine loses its dissolving power when it meets such large proportions of silver.
The curves bring out the additional interesting fact that changes in the degree of concentration of chlorine, as well as of bromine, do not make a very decided difference in the percentage of extraction with pure gold or gold with 10 per cent, of silver, as long as with 150 c.c. of water, 1.4 g. of chlorine, equivalent to 3 g. of bromine, is not overstepped as the lower limit. With gold containing more than 10 per cent, of silver, a slight decrease in the concentration makes a very decided difference in the extraction until 1.2 g. is reached with chlorine, and 2.1 g. with bromine. If the solutions are further diluted, the effect of an increase in silver is not so very marked.
The Effect of Silver on the Chlorination and Bromination of Gold., BY H. 0. HOFMAN
