Gold can alloy with almost all other metals, but most of the bodies thus formed are of little or no practical importance. Tin, zinc, arsenic and antimony unite with gold with contraction, and form pale yellow or grey coloured, hard, brittle and easily fusible alloys, of which all, except those containing zinc, are soluble with difficulty in aqua regia. The arsenic and antimony alloys are slowly decomposed by mercury, the base metal being separated as a black powder, which consists in part of arsenide or antimonide of mercury. Lead and iron alloy with gold with expansion, while in the case of copper no change of volume takes place.

Gold alloys with a small percentage of lead is a hard, brittle, pale-yellow substance, which can be crumbled with the fingers. If more than about 4 per cent, of lead is present, there is marked segregation on solidification, and this also takes place in the case of the zinc alloy and of some others.
Heycock and Neville have shown that the freezing point of lead is lowered by the addition of gold to it in accordance with the general law. Thus, the freezing point of pure lead being 327°, an addition of 3.8 per cent, of gold reduces it to 301°, and Roberts-Austen has recently found that the eutectic alloy of gold and lead, which contains about 13 per cent, of the former metal, melts somewhere between 190° and 198°. Similarly, by adding 6.9 per cent, of gold to thallium, the freezing point of the latter is lowered from 301° to 261°.
Several alloys of gold with other metals in molecular proportions have been isolated, or their existence proved in various ways. Thus, for example, the compound AuSn was recognised by Mathiessen, from the curve of electric conductivity of the gold-tin series of alloys, and this substance was more recently detected by A. P. Laurie by observing the electro-motive force developed by alloys of different compositions when dipped into a solution of SnCl2. Heycock and Neville succeeded in isolating the compound AuCd in 1892, after having suspected its existence for some time. In the same year, Mylius and Fromm prepared a number of gold alloys by precipitation from solution. Thus a gold-zinc alloy, containing equal weights of the two metals, and approximating in composition to AuZn3, was obtained in the form of black, spongy flocks, by adding a solution of gold sulphate to water in which a zinc plate was placed. The gold- zinc slimes obtained in the cyanide process (q.v.) may be compared with this. Gold-cadmium, similarly obtained, is a lead- grey crystalline precipitate, having the composition AuCd3. On heating, it is converted into gold-mono-cadmium, AuCd (see above). If the gold-zinc alloy is shaken with a solution containing a cadmium salt, the gold-cadmium alloy and a zinc salt are obtained. Similarly, a copper plate, acting on solutions containing gold, yields a black, spongy compound of gold and copper; and gold-lead and gold-tin alloys in the form of black slimes are also readily prepared.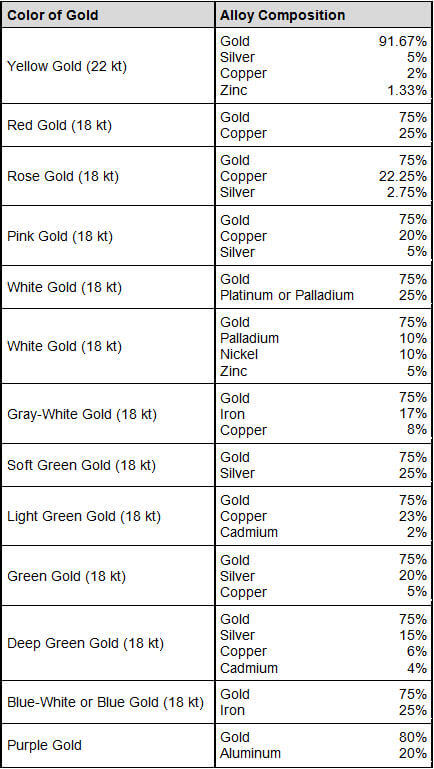
The aluminium alloys have been investigated by Roberts- Austen. These alloys are remarkable for their intense colour, varying from yellowish-green to purple, and some of them appear to present the characteristics of true chemical compounds. A white alloy containing 10 per cent, of aluminium is very hard, and has a melting point no less than 417° lower than that of pure gold; but a deep purple alloy, containing 22 per cent, of aluminium (corresponding to the formula AuAl2), appears to melt at a temperature between 1,065° and 1,070° , or over 20° higher than the melting point of pure gold. It presents, therefore, the extremely rare case of an alloy, the fusing point of which is higher than that of the least fusible of its constituents, and this fact affords strong evidence that it is a true compound of gold and aluminium. It is hardly necessary to point out that the melting points of ordinary chemical compounds are often much higher than the melting point of the least fusible constituent. There is a strong tendency for this purple alloy to be formed when gold is melted with an excess of aluminium, and the result is that the alloys rich in aluminium usually show a marked lack of homogeneity. Evidence of the existence of the compound AuAl2 was also obtained by Heycock and Neville by noting the effect on the freezing point of tin caused by additions of gold and aluminium in various proportions. A crystalline alloy of gold and bismuth, containing gold 68.22 per cent., bismuth 31.78 per cent., has been prepared by Pearce by means of liquation. By melting this with silver, a crystalline alloy corresponding to the formula AuAg was produced, and crystalline alloys of gold, silver and copper were also obtained. Specimens of these products are in the Percy collection.
The diffusion of gold into other metals, both liquid and solid, has lately been investigated by Roberts-Austen. The rates of diffusion in liquid metals are of the same order as those of soluble salts in water, but the diffusion in solids is very slow. If gold is placed at the base of a cylinder of solid lead 70 mm. high, some is found to have reached the top in thirty days, the temperature being kept at 251°. The rate of diffusion is still measurable at 100°, but is almost inappreciable at ordinary temperatures.
The alloys which are most important to the metallurgist are those which gold forms with mercury, copper, silver, lead, and zinc.
Liquation of Gold Alloys
The subject of liquation generally, including that observed in gold alloys, has been discussed in the volume introductory to this series. Experiments of Roberts-Austen and of Peligot are there described which tend to prove that liquation does not occur in the gold-silver and gold-copper alloys rich in gold and free from all impurities. Levol had previously come to the same conclusion as Peligot with regard to gold-copper alloys, but gave no details of his experiments, remarking, however, that the oxidation of the copper made the research difficult. Roberts-Austen has also cited the evidence afforded in the preparation of the standard gold trial plate made in the Royal Mint as conclusive in proving that considerable masses of standard gold can be obtained of uniform composition. A mass of standard gold weighing 72 ozs. was cast in a suitable mould, and rolled into a plate 37 inches long and 6.5 inches wide; portions of metal were cut from different parts of it, and when these were assayed it was found that the greatest variation between any two assays was 2/10000, there being no evidence of concentration of the precious metal anywhere. In this case the gold and copper used to make the alloy were of exceptional purity. In 1889, moreover, this chemist examined the composition of two large ingots, weighing 400 ozs. each, which had been sent to the Mint for coinage by the Bank of England. The fineness of these ingots was 896.2 and 978.5 respectively, and the results showed that no definite liquation had taken place in them. In the case of many of the ordinary trade ingots, however, the discrepancies between assays on pieces taken from opposite ends of the bars prove that the composition is not uniform, but this lack of uniformity is doubtless due to the presence of some other element in addition to gold, silver and copper.
- Parting gold with Nitric Acid—Experimental Work.
- Gold Parting with Silver as the Alloying Metal.
- Gold Parting with Zinc as the Alloying Metal.
- Gold Parting with Sodium as the Alloying Metal.
- Parting Gold with Nitric Acid on a Large Scale.
James C. Booth, of the U.S. Mint, Philadelphia, has stated that liquation of gold-copper alloys is induced by the presence of small quantities of base metals, the greatest effect being produced by antimony, bismuth and arsenic, while lead, tin and zinc act in the same way to a less degree, but no direct experiments have been adduced in support of this view. Alloys containing much lead, bismuth and zinc cannot be obtained perfectly homogeneous.
It has been pointed out by the author that segregation takes place in standard gold rendered crystalline by small quantities of lead or bismuth, the presence of 0.2 per cent, of either of these metals causing the centre of a sphere 3 inches in diameter to be enriched in gold to the extent of about one part per thousand. This is due to the fact, proved by Roberts-Austen, that an eutectic alloy of gold and lead remains molten after the remainder of the mass has solidified, and is consequently driven towards the centre of the sphere, and that this fusible alloy contains much gold but very little copper. Arnold’s micro graphic results tend to confirm the view which follows from this that the brittleness of crystalline gold is due to the presence of films composed of such eutectic alloys separating the crystals of gold from each other, but Osmond and Roberts-Austen proved the non-existence of these films in quickly-cooled ingots.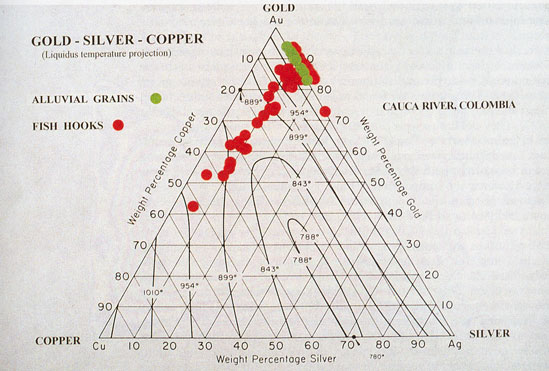
It has been shown by Edward Matthey that when gold ingots containing members of the platinum group are cooled from a state of fusion an alloy rich in the more fusible element (gold) falls out first, driving the less fusible constituent to the centre. Thus the assay of an outside cut of such an ingot gives a result too high in gold, sometimes by several per cent. It has long been known, moreover, that iridium and osmium become concentrated towards the bottom of the mass.. The reason for this is that, at the temperature of fusion of gold, these refractory elements, either free or alloyed with gold, sink in the molten metal and are left in the state of small crystalline particles.
Matthey has more recently investigated the question of segregation in connection with the alloys of gold, silver, lead and zinc produced in cyanide mills. He found that one such ingot weighing about 120 ozs. contained 662 parts per 1,000 of gold at the bottom corner, and only 439 parts at the top. In another case, when 16.4 per cent, of lead and 9.5 per cent of zinc were present, the standard fineness of an ingot weighing 400 ozs., as shown by actually separating the whole of the precious metals, was, gold 614.0, silver 75.8, and its true value £1,028, while the value as deduced from the average of fourteen assays made on it (gold 576.0, silver 90) would have been only £965. Seven dip assays made on this ingot varied from 562 to 622 fine. Other cases of irregular distribution were even more remarkable. By experiments with synthetic alloys of gold and zinc, Matthey found that gold tends to liquate towards the centre of the mass but only in a slight degree, the centre of a 3-inch sphere of an alloy containing gold 90, zinc 10, being only about 1 to 2½ parts per 1,000 richer than the outside. Lead acts similarly but with greater effect, the centre being about 29 parts per 1,000 richer in gold than the outside when 30 per cent, of lead is present, but the combination of 15 per cent, of lead and 10 per cent, of zinc is still more powerful in causing segregation, the sphere being found to contain 657 parts of gold at the top, 785 in the centre, and 790 at the bottom, gravity thus playing a part. The addition of silver, however, if it amounts to not less than about two-thirds of the quantity of zinc and lead taken together, appears absolutely to prevent any liquation from taking place, an alloy containing approximately, gold 55, zinc 7, lead 18, silver 20, being practically homogeneous.
https://www.totalmateria.com/page.aspx?ID=CheckArticle&site=ktn&NM=230
Gold can be made to alloy with almost all other metals, but most of the bodies thus formed are of little or no practical importance. Tin, zinc, arsenic and antimony unite with gold with contraction, and form pale yellow or grey coloured, hard, brittle and easily fusible alloys, of which all, except those containing zinc, are soluble with difficulty in aqua regia. The arsenic and antimony alloys are slowly decomposed by mercury, the base metal being separated as a black powder, which consists in part of arsenide or antimonide of mercury. Lead and iron alloy with gold with expansion, while in the case of copper no change of volume takes place.
Gold alloyed with a small percentage of lead is a hard, brittle, pale-yellow substance, which has a musical ring. If more than about 4 per cent, of lead is present, there is marked segregation on solidification, and this also takes place in the case of the zinc alloy and of some others.
Heycock and Neville have shown that the freezing point of lead is lowered by the addition of gold to it in accordance with the general law. Thus, the freezing point of pure lead being 327°, an addition of 3.8 per cent, of gold reduces it to 301°, and Roberts-Austen found that the eutectic alloy of gold and lead, which contains about 13 per cent, of the former metal, melts somewhere between 190° and 198°. Similarly, by adding 6.9 per cent, of gold to thallium, the freezing point of the latter is lowered from 301° to 261°.
Several compounds of gold with other metals have been isolated, or their existence proved in various ways. Thus, for example, the compound AuSn was recognised by Matthiessen, from the curve of electric conductivity of the gold-tin series of alloys, and this substance was more recently detected by A. P. Laurie by observing the electro-motive force developed by alloys of different compositions when dipped into a solution of SnCl2. Heycock and Neville succeeded in isolating the compound AuCd in 1892, after having suspected its existence for some time. In the same year, Mylius and Fromm prepared a number of gold alloys by precipitation from solution, Thus a gold-zinc alloy, containing equal weights of the two metals, and approximating in composition to AuZn3, was obtained in the form of black, spongy flocks, by adding a solution of gold sulphate to water in which a zinc plate was placed. The gold-zinc slimes obtained in the cyanide process (q.v.) may be compared with this. Gold-cadmium, similarly obtained, is a lead-grey crystalline precipitate, having the composition AuCd3. On heating, it is converted into gold-mono-cadmium, AuCd (see above). If the gold-zinc alloy is shaken with a solution containing a cadmium salt, the gold-cadmium alloy and a zinc salt are obtained. Similarly, a copper plate, acting on solutions containing gold, yields a black, spongy compound of gold and copper; and gold-lead and gold-tin alloys in the form of black slimes are also readily prepared.
The diffusion of gold into other metals, both liquid and solid, was investigated by Roberts-Austen. The rates of diffusion in liquid metals are of the same order as those of soluble salts in water but the diffusion in solids is very slow. If gold is placed at the base of a cylinder of solid lead 70 mm. high, some is found to have reached the top in thirty days, the temperature being kept at 251°. The rate of diffusion is still measurable at 100°, but is almost inappreciable at ordinary temperatures.
The alloys which are most important to the metallurgist are those which gold forms with mercury, copper, silver, lead, and zinc.
Gold and Aluminium
These alloys were investigated by Roberts-Austen, and more recently by Heycock and Neville. Several true compounds of the two metals were proved to exist, the most remarkable and stable being Au2Al, a hard white alloy with a freezing point of only 622°, and AuAl2, Roberts-Austen’s beautiful purple alloy which melts at a higher temperature than the fusing point of pure gold. This compound is always formed when mixtures of gold and aluminium are fused, and allowed to cool, provided that not more than about 90 per cent, of gold is present. The purple alloy is seen in patches on a white ground containing the excess of aluminium or of white compounds of gold and aluminium.
Amalgams.—A piece of gold rubbed with mercury is immediately penetrated by it and becomes exceedingly brittle. The ductility is not always restored when the mercury is removed by distillation, a crystalline structure being often induced, perhaps because part of the mercury is retained by the gold. A particle of gold “ wetted ” by mercury at once loses its colour. A solid amalgam is formed, but is not readily dissolved in an excess of mercury. Kasentseff has shown that mercury dissolves 0.11 per cent, of gold at 0°, 0.126 per cent, at 20° and 0.65 per cent, at 100°. The mercury in a mill in time becomes a saturated solution of gold, containing say 0.12 per cent, and the amalgams, which may be regarded as solutions of mercury in gold, do not mix with the mercury, but sink to the bottom if left undisturbed. By filtering, the amalgam can be removed, the liquid solution passing through the filter bag.
The composition of a saturated solution of mercury in gold is not known. It probably contains about 85 per cent, of mercury and 15 per cent, of gold. This saturated solution is solid, and is “ wetted ” by fresh mercury, but if more mercury is added the excess can be removed by filtering under pressure. Pasty amalgam is left in the filter bag, and becomes “drier” and less pasty the more the pressure is increased.
The composition of the amalgam recovered in mills in this way varies considerably. Coarse particles of gold are not saturated with mercury to their centres, although the outside layers of the particles consist of a saturated solution. The saturation of the interior, depending on the diffusion of mercury through solid amalgam, would probably not be complete for many days. The finer (i.e., smaller) the particles of gold, the more nearly the interior approaches to saturation. It follows that coarse gold gives rich amalgam, and fine gold poor amalgam. The limits are pure gold on the one hand and a saturated solution of mercury in gold on the other, neither of which exist in practice. The practical limits are amalgams containing about 50 per cent, and 25 per cent, of gold respectively. These gold amalgams usually contain impurities in the shape of amalgams of silver and of base metals, as well as non-metallic substances. The actual value of squeezed amalgam consequently varies from £2 to a few shillings per ounce.
When amalgams are gradually heated, the mercury is distilled off by degrees, the action soon ceasing if the temperature is allowed to become stationary, and distillation recommencing if it is again raised. At 440° (somewhat below a red heat), an amalgam containing about three parts of gold to one of mercury is obtained, and at a bright red heat almost all the mercury is expelled, and if the heating has not been pushed too rapidly the vapours contain but little gold. The gold obstinately retains about 0.1 per cent, of mercury, which is not driven off below the melting point of gold.
Gold and Silver
Gold and silver unite in all proportions, forming solid solutions (or isomorphous mixtures) with properties intermediate between those of the two metals. The first additions of silver do not lower the melting point of gold, and until silver forms 35 per cent, of the alloy, little change in the melting point occurs. The first additions of gold to silver raise its melting point. The alloys are homogeneous, malleable, soft, and ductile. They are suitable for the material of trial-plates, as they are uniform in composition. The colour of gold is sensibly lowered by the addition of very small quantities of silver, and, on increasing the proportion of the latter, the colour changes by tints of a greenish-yellow (when from 20 to 40 per cent, of silver is present) to white, with a scarcely perceptible yellow tinge (when 50 per cent, of silver is present), and silver-white (when more than 60 per cent, of silver is present). The alloys containing small quantities (less than 20 per cent.) of gold are said to separate by gravity if kept for some time in a state of quiet fusion, an alloy containing one part of gold to five parts of silver (AuAg9) sinking to the bottom, and slightly auriferous silver floating at the top. The silver-gold alloys most used in jewellery are green gold (silver 25, gold 75), dead-leaf gold (silver 30, gold 70), and the alloy containing 40 per cent, of silver. Triple alloys of gold, silver, and copper are employed far more frequently by English jewellers than those last mentioned; of these the alloys, consisting of 22-, 18-, 15-, 12-, and 9-carat gold respectively, can be hallmarked, Alloys of gold and silver were much used for coinage before the methods of parting became well known and inexpensive.
Electrum includes pale yellow alloys with from 15 to 35 per cent, of silver. It occurs native, and was much used for ornaments and coins by the Greeks and Romans, and by the nations which acquired their arts. The use of silver in the gold- copper coinage alloys was not discontinued until quite recently, all English guineas and the Australian sovereigns manufactured at Sydney up to the year 1871 containing some of it. Both nitric and sulphuric acids attack silver-gold alloys, almost completely dissolving out the silver if it is present in amounts variously stated at from 60 to 70 per cent., while, if the proportion falls below 60 per cent., some of the silver is left undissolved with the gold. Hydrochloric acid scarcely attacks these alloys, and the action of aqua regia is soon arrested if the proportion of silver is considerable. They may be dissolved by a mixture of nitric acid and a concentrated solution of common salt.
The densities of the alloys of silver and gold are as follows :—
Gold and Copper
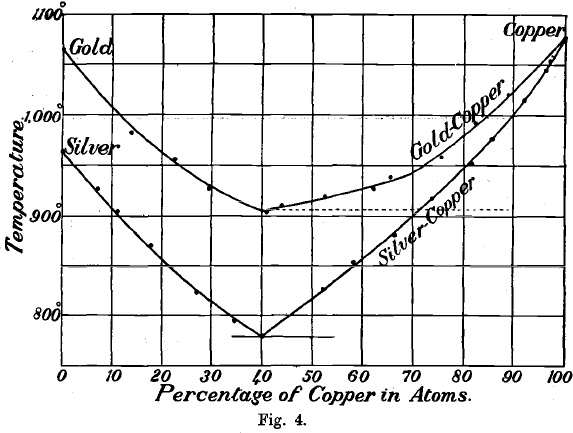
It was long believed that these metals dissolve in one another in all proportions, forming a complete series of homogeneous alloys. It has been shown, however, by an examination of the freezing point curves and the microscopic structure of the series of alloys, that they closely resemble the silver-copper series. The freezing point curves of the two series reckoned in atomic percentages are shown in Fig. 4. From this it is clear that the alloys having the lowest freezing point in each series contain 40 atoms of copper mixed with 60 atoms of gold and silver respectively. Microscopic examination proves that both these alloys are true eutectics showing the characteristic banded structure. Both are the only brittle members of their series, breaking with conchoidal fracture, and are the only homogeneous alloys of gold and copper and of silver and copper.
It had previously been pointed out by the author that segregation takes place in standard gold rendered crystalline by small quantities of lead or bismuth, the presence of 0.2 per cent, of either of these metals causing the centre of a sphere 3 inches in diameter to be enriched in gold to the extent of about one part per thousand. This is due to the fact, proved by Roberts- Austen, that a eutectic alloy of gold and lead remains molten after the remainder of the mass is solidified, and is consequently driven towards the centre of the sphere, and that this fusible alloy contains much gold but very little copper. Arnold’s micrographic results tend to confirm the view which follows from this that the brittleness of crystalline gold is due to the presence of films composed of such eutectic alloys separating the crystals of gold from each other, but Osmond and Roberts-Austen proved the non-existence of these films in quickly-cooled ingots.
Standard gold, however, is made too brittle for coinage by the presence of a far smaller amount of lead than 0.2 per cent. The addition of 0.05 per cent, of lead produces bars almost as brittle as those containing 0.2 per cent., and from researches in the Royal Mint, it would appear that 0.01 per cent, of lead or even less renders gold unfit for coinage.
The alloys of gold and copper are less malleable, harder, and more elastic than gold, and possess a reddish tint. Those with less than 12 per cent, of copper are fairly malleable; when more than this is present they are difficult to work owing to their hardness. Since no change of volume occurs when these alloys ; are formed, their densities may be calculated from those of gold and copper. The densities of gold-copper alloys are as follows :—
The density of standard gold is usually given as 17.48, and that of the alloy containing gold 900, copper 100 as 17.16. For the densities of other gold-copper alloys, see Rigg in the Report of the Mint, 1876, p. 46. Many of the alloys have been used for coinage at various times. The Greeks and Romans, after electrum had fallen into disuse, employed the purest gold they could procure, viz., that from 990 to 997 fine. Under the Roman Emperors, however, copper was intentionally added, and in the two centuries preceding the fall of Rome very base alloys were used, some containing only 2 per cent, of gold or even less. In the middle ages these base alloys were discarded, and the “byzant” of Constantinople and the “florin” of Florence were both nearly pure gold, while the first gold coins struck by the nations of Western Europe were also intended to be absolutely fine. The standard 916.6 or 11/12 (i.e., 916.6 parts of gold in 1,000) was adopted by England in the year 1526, the standard of 994.8, which had been introduced in 1343, being finally abandoned in 1637 ; the 900 standard was introduced in France in 1794, and subsequently adopted in other countries. These two standards are now those most commonly used, the English standard being employed by Russia, Portugal, India, and Turkey, and the French standard by most other civilised countries; the Austrian ducat, however, has a fineness of 986 and that of Holland a fineness of 983, while the Egyptian standard is only 875. Of all these alloys the 900 and 916.6 standards are those best adapted for coinage, keeping their colour fairly well, and resisting wear better than richer alloys. The 900 alloy is harder and wears better than the 916.6 alloy, but the difference is not great, the rate of wear depending less on such small differences of composition than on the mechanical and thermal treatment of the alloys during the operation of coining. The alloys used in coinage generally contain from two to twelve parts of silver per 1,000 in addition to the gold.
Gold-copper alloys tarnish on exposure to air owing to oxidation of the copper, and blacken on heating in air from the same cause. This oxidised coating may be removed and the colour of fine gold (not that of the original alloy) produced by plunging the metal into dilute acids or alkaline solutions, the operation being technically known as “ blanching.” The colour of alloys may be improved without previous oxidation by dissolving out some copper by acids, a film of pure gold being thus left on the outside which can be burnished. French jewellers use a hot solution of two parts of nitrate of potash, one part of alum, and one of common salt for this purpose.
Nitric or sulphuric acid dissolves out the copper from gold- copper alloys under conditions similar to those under which it removes silver from silver-gold alloys. If the copper falls below 6.5 per cent, the alloy is not attacked by these acids (Pearce). Aqua regia dissolves all the alloys completely.
Liquation of Gold Alloys
The subject of liquation generally, including that observed in gold alloys, has been discussed in the volume introductory to this series.
Liquation in the case of gold-copper alloys has been discussed above, p. 17. It has been shown by Edward Matthey that, when gold ingots containing members of the platinum group are cooled from a state of fusion, an alloy rich in the more fusible element (gold) falls out first, driving the less fusible constituent to the centre. Thus the assay of an outside cut of such an ingot gives a result too high in gold, sometimes by several per cent. It has long been known, moreover, that iridium and osmium become concentrated towards the bottom of the mass. The reason for this is that, at the temperature of fusion of gold, these refractory elements, either free or alloyed with gold, sink in the molten metal and are left in the state of small crystalline particles.
Matthey has more recently investigated the question of segregation in connection with the alloys of gold, silver, lead and zinc produced in cyanide mills. He found that one such ingot weighing about 120 ozs. contained 662 parts per 1,000 of gold at the bottom corner, and only 439 parts at the top. In another case, when 16.4 per cent, of lead and 9.5 per cent, of zinc were present, the standard fineness of an ingot weighing 400 ozs., as shown by actually separating the whole of the precious metals, was, gold 614.0, silver 75.8, and its true value £1,028, while the value as deducted from the average of fourteen assays made on it (gold 576.0, silver 90) would have been only £965. Seven dip assays made on this ingot varied from 562 to 622 fine. Other cases of irregular distribution were even more remarkable. By experiments with synthetic alloys of gold and zinc, Matthey found that gold tends to liquate towards the centre of the mass, but only in a slight degree, the centre of a 3-inch sphere of an alloy containing gold 90, zinc 10, being only about 1 to 2½ parts per 1,000 richer than the outside. Lead acts similarly, but with greater effect, the centre being about 29 parts per 1,000 richer in gold than the outside when 30 per cent, of lead is present, but the combination of 15 per cent, of lead and 10 per cent, of zinc is still more powerful in causing segregation, the sphere being found to contain 657 parts of gold at the top, 785 in the centre, and 790 at the bottom, gravity thus playing a part. The addition of silver, however, if it amounts to not less than about two-thirds of the quantity of zinc and lead taken together, appears absolutely to prevent any liquation from taking place, an alloy containing approximately, gold 55, zinc 7, lead 18, silver 20, being practically homogeneous.
