Friends often ask how accurate the work of our little stamp-mill is, or express the opinion that a little mill cannot do good work. As a reply we would like to place on record the results of a few tests.
There are two periods of work in our laboratory; the fall term, when the class collectively goes through all our chief laboratory-processes, and the spring term, when the students work up their theses, each electing some one process or investigation and devoting himself wholly, or almost wholly, to that.
Some of the tests, the results of which are recorded in this paper, were made in the fall term; the preparation of the apparatus, the sampling and the assaying being done largely by assistants, with the aid of the students. Others were made in the spring term as thesis-work, and the results are due wholly to the students.
The complete “ tree ” of our process is patterned after the most approved California mill-practice. It is modified only where the small-scale method must differ from the large, in order to secure results from a brief test lasting a few hours, followed, perhaps, by an interval of many months between tests, —which seek to rank in accuracy with those of the large-scale work, running over months and years of continuous work, broken only by brief intervals for cleaning-up.

We use no battery-plates, because we believe the loss due to absorption of gold by the copper may be greater than the gain from using them.
We use silver amalgam on our apron-plate, painting it on before the run, and scraping it off with the gold-contents afterwards, for two reasons: It renders the plate very quick to catch gold, and it renders the absorption-loss by the copper-plate insignificant, or prevents it altogether.
Our canvas and fine vanner-plant for treating the extremely fine tailings is that known as the Gates canvas-plant, originally designed by G. G. Gates and installed at the Kennedy mine, Jackson, California. Since it has been demonstrated clearly that this canvas-plant can pay only when the assay of concentrates is high, or when the percentage of concentrates is large, we do not always choose this run as the one to illustrate to students the wonderful catching-power of canvas for extremely fine concentrates. We do, however, make it our practice to separate, by classifying, the coarse Frue-vanner tailings from the fine, thereby preparing for canvas should we decide to use it.
What is Free milling: Ores of gold or silver from which the precious metals can be recovered by concentrating methods without resorting to pressure leaching or other chemical treatment.
Sampling.—Many of the ores we run would come under the head of “ rich spotty ” ores, as the Colorado phrase is. To crush and cut down such an ore, according to best Colorado rules of sampling, would require breaking the whole batch of from 500 to 1,000 lb. down to 10 or even 30-mesh. This would ruin our gold stamp-mill run. We are thus put in the odd position of having to choose between sampling the lot according to the best rules for safe work and spoiling the stamp-run, or of making a stamp-mill run, using only a very crude sample for valuation, which we could not possibly defend on the score of accuracy. It is needless to say we choose the latter method.
The mill and vanner were run under the following conditions :—
Apron-plate of copper 6 ft. long, 2 ft. wide, covered with silver amalgam ; number of stamps, 3; weight of stamps, 225 lb.; diameter of shoe, 5.75 in.; number of drops, 98 per min.; height of drop, from 4.25 to 5.75 in.; height of discharge, 4.75 in.; screen, horizontally slotted, openings 1/40 in. wide; rate of crushing, 57 to 94 kilos, per hour; plate-slope, 1.5 in. per ft.; feed-water to the battery, 15 kilos, per min.; wash-water on vanner, from 4 to 8 kilos, per min.; Frue vanner-slope, 3.5 to 4.5 in. in 12 ft.; number of shakes, 198 per min.; belt-travel, from 40 to 60 in. per min.; canvas-table, 10 ft. long, 4 ft. wide; canvas-table slope, 1.5 in. per ft.; canvas-table feed, 10 kilos, water per min. and from 0.7 to 0.8 kilos, dry ore per min.; Embrey vanner, 1.5 in. per ft. slope, 80 in. per min. travel of belt, 1240 vibrations of 0.5 in. per min.
The mill is a little 3-stamp Fraser and Chalmers prospecting-mill of 1882. It is very satisfactory in all respects except the number of stamps. A school-battery should have either two or five stamps; the former is preferred by us. Three stamps cannot have an order of drop which satisfactorily distributes the sand.
The vanner is a standard 4-ft. Frue, made by Fraser and Chalmers in 1882. It is a very good one; the belt being today as good as new. It is run from 5 to 10 days in a year.
The canvas-table is supplied with an accurate distributer. The canvas is No. 6 cotton duck, with the warp laid across the table for the greatest roughness, and is cut from a roll woven 10 ft. wide. The feed-water is the quantity, and the pulp is two to three times the quantity used by the Gates canvas-plant. The copper-plate is 1/8 in. thick, of softest copper.
The amalgam is made by dissolving 95 grammes silver in 380 c.c. nitric acid (1.2 sp. gr.) with addition of 817 c.c. water after solution. Pure mercury is then added at the rate of 16 parts mercury to 1 part silver. It should be stirred at short intervals during the formation of the amalgam. The result is a soft amalgam, clean and free from crystals or mercurous nitrate. Failure to follow this rule exactly is liable to give a very impure amalgam, which will require time and trouble to clean.
The apron-plate is cleaned preparatory to the application of silver amalgam as follows: It is washed off with water, scoured wet with infusorial earth or very fine sand, and then washed off with water again. Obstinate black spots can be removed by scouring wet with a flat piece of pumice-stone, adding mercury to plate the cleaned copper. It is then washed all over with a strong solution of cyanide, adding mercury, again rinsed off with water, and then rubbed dry with clean cloth or cotton- waste which must be free from oil. A moderate quantity of mercury is now rubbed on without water, after which the plate is ready for the amalgam. No water is allowed upon it yet.
The clean amalgam is squeezed in chamois skin to a moderately hard ball, and rubbed without water on the clean, bright, amalgamated copper-surface of the apron-plate. (A flat, bristle paste-brush may be useful to obtain an even distribution.) This coating is quick to catch gold, and prevents the gold from coming in contact with the copper. No water is allowed upon it until stamping begins.
After the run, the plate is rinsed off with water, and the amalgam scraped off as clean as possible with a rubber-scraper. More mercury is rubbed on, and the plate is again scraped. This is repeated twice more, and all the amalgam is put together. If there is any hard amalgam which has not been removed, it is gently rubbed wet with a flat piece of pumice-stone in the presence of mercury, and so removed without injury to the plate. The amalgam from the plate, combined with that from the traps, is retorted; the residue is melted with borax in a crucible, and the bullion is parted for gold.
The battery-residue is worked up, as shown in the tree of the process, yielding amalgam, Wilfley heads, coarse tailings and fine tailings, which are added to the main portions of these products.
Since the battery is fed with pure mercury, while the apron-plate is coated with silver amalgam, the battery-amalgam enables us to get a valuation on the fineness of the gold caught at this point. We have no means to get this ratio on the portion of the gold caught on the apron-plate.
The mercurous nitrate and a little residual silver nitrate from making silver amalgam are worked over for mercury and silver by immersing strips of iron in the liquid, which precipitates the metals as amalgam.
The results of seven tests are here given.












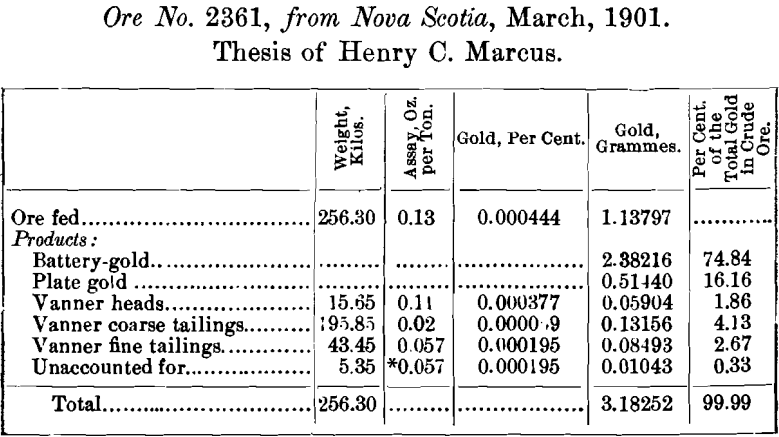


Attention is called to the slight variation in the mill-assay of the four tests on No. 2444, and also to the considerable variation in the assays by fire.
The method here used in tabulating the results of a test has been found to be a most concise and satisfactory one. As the value of the ore obtained by the mill-run is much more accurate than that based on the assay of the ore, the percentages found in the column headed “ Per cent, of the total gold ” are based on the total gold in the ore as found by the mill-run, and not on the assay of the feed; in fact, one of the objects of this work is to show the student the impossibility of valuing a free-milling gold-ore by sampling and assaying.
The degree of accuracy attained here is, we believe, up to that of the very best mill-practice. It has been attained by locating the causes of error, and then by removing them as completely as possible, even by departing, if necessary, from large-scale mill-methods.
