Fluorspar or Fluorite; CaF2. — Color, white, yellow, green, red, blue, deep purple, sometimes brown; powder, white; luster, glassy; H = 4; G = 3.01 to 3.25; crystals, in cubic system, mostly cubes; cleavage, perfect, cleavage pieces tending to show equilateral triangles which are also often to be seen outlined by cracks and edges; composition, calcium and fluorine, forming calcium fluoride.
Used principally as a flux in smelting, particularly in the manufacture of iron and steel; in the manufacture of enamels and opaque glass; in the manufacture of hydrofluoric acid, used in etching glass; to make artificial cryolite, for use in the electric furnaces that make aluminum. Very perfect, colorless crystals (optical spar) are valuable for use in making certain optical instruments. Some colored varieties (Derbyshire blue john) are used for ornamental vases.
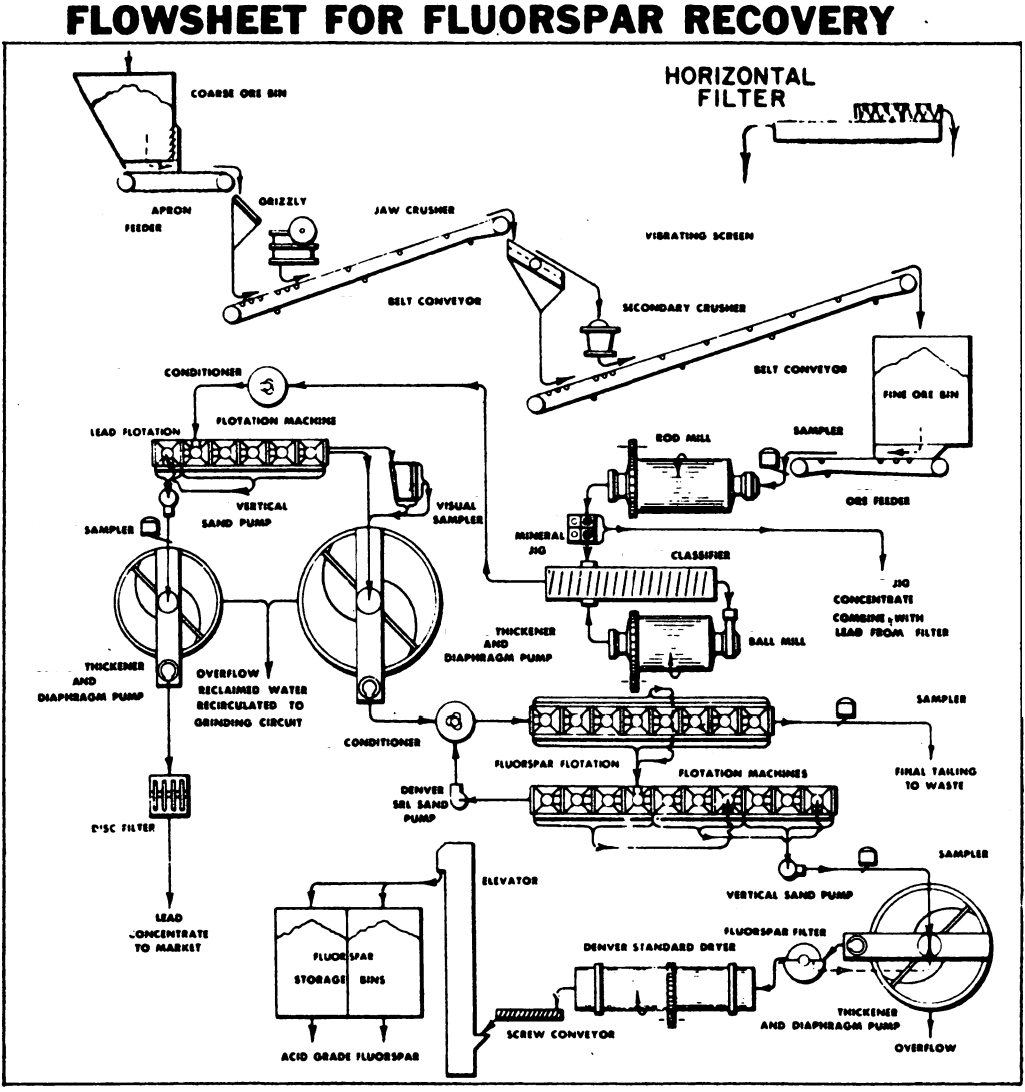
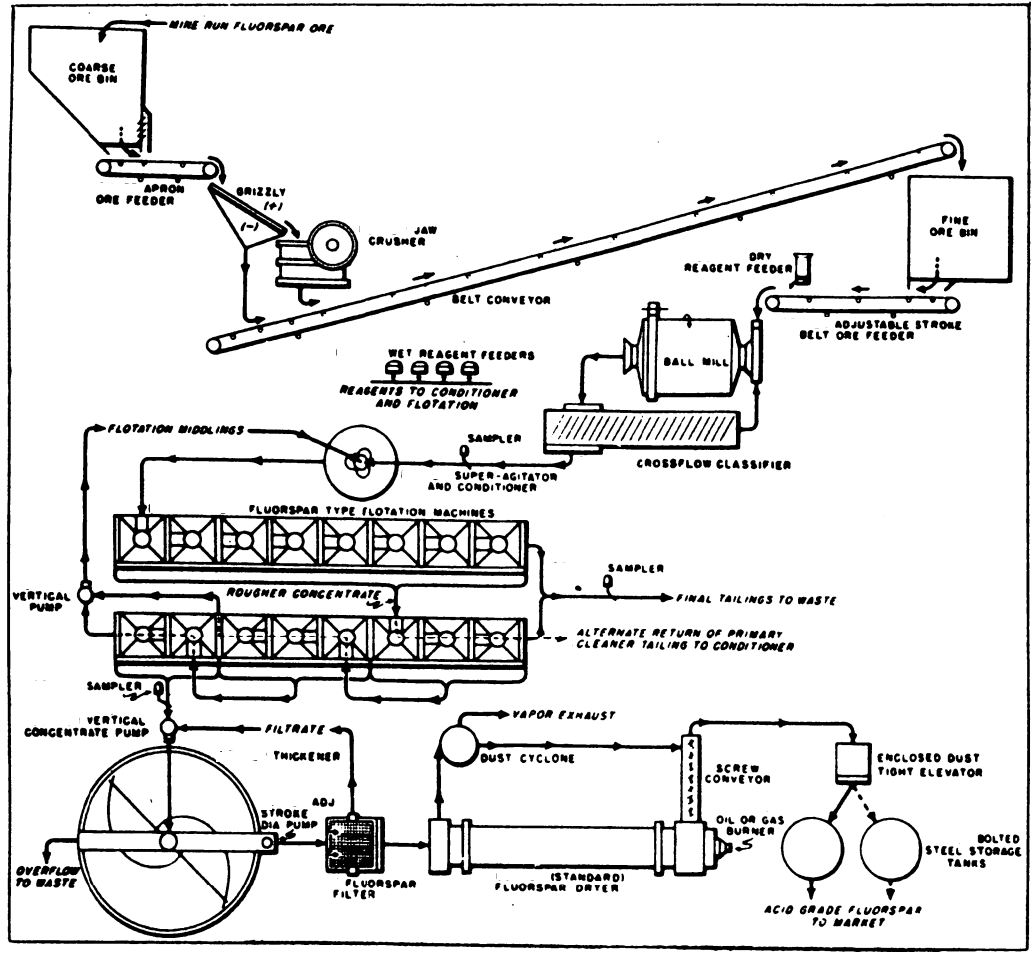
Value, lump, containing not less than 85% calcium fluoride and not over 5% silica, $25 to $35 per ton. Best quality, for manufacture of hydrofluoric acid, $50 per ton. Fluorspar is found generally in veins, in gneiss, slate, limestone, crystalline limestone, and sandstone; often as gangue of metal ores, particularly of lead ores. It is sometimes mixed with barite and celestite; and as these minerals are partly sulphur in composition, they lower the value of fluorspar, as sulphur is injurious to iron and steel.