The pulping consisted of disintegration of the wastepaper structure and dispersion of the fibers. The disintegration was accomplished by conditioning 250 grams of wastepaper, under moderate agitation, with hot steam (84 to 90 °C), de-ionized water and reagent as desired. After disintegration the pulp was further dispersed in a high speed blender. All pulping experiments were done at a consistency of approximately 12% solids by weight.
Pulping for all flotation de-inking was carried out under identical conditions to produce the same degree of dispersion prior to flotation. It should be noted that this is not necessarily the conditions for optimal ink removal. The pulped wastepaper was diluted in a four liter flotation cell to a consistency of 1 % and conditioned for 2 minutes in the presence of the collector LIONSURF 768 (0.4 pounds/ton of dry paper) as provided by Lion Industries. LIONSURF 768 is a nonionic surfactant/fatty acid blend. The flotation was then carried out for 15 minutes at a stirring speed of 1400 rpm. Subsequently, the float (toner particles) and non-float (cellulose fibers) product were carefully filtered, dried and stored for analysis.
Figure 3 shows the flotation de-inking response for alkaline pulping with the removal of toner and mineral fillers (kaolinite (Al) and anatase (Ti)) at different pH values. As can be noted, the removal of toner, anatase and kaolinite as a function of pH is similar and goes through a maximum at pH 6.3.
In the case of the acid pulping, Figure 4, the overall removal of toner and anatase particles improves considerably when compared to the results for alkaline pulping. Once again the maximum removal is achieved at pH 6.3.
The inefficient ink removal for the alkaline pulping case can not only be related to the larger toner particle size but also to their surface properties. Recently, contact angle measurements have been reported for laser printed toner films treated with caustic solution at high temperature (Drelich et. al., 1996). On the basis of these measurements, it is known that both elevated temperature and alkaline conditions significantly change the wettability of the toner particles. The alkalinity promotes a drastic decrease in the contact angle from 105-115 degrees to 75-80 degrees. Therefore, the caustic pulping environment not only promotes the release of larger toner particles but also reduces their hydrophobicity.
Independent of the pulping conditions the toner removal increases for flotation under acid conditions. The reason for the improvement is uncertain. There is no reversibility of the toner surface charge with pH (PZC ~2). Of course under acid conditions the collector (fatty acid) species is no longer dissociated, but rather is present in the acid form and may even precipitate as colloidal droplets. It appears that adsorption of the acid form may account for the increase in the hydrophobicity of the toner surface and consequently enhance its removal by flotation. However such explanation does not account for the decrease in flotation below pH 4.5 under alkaline pulping conditions.
Acid pulping not only increases the removal of toner but also the removal of the mineral fillers. The explanation for improved removal of mineral filler can be related to the fact that acid conditions may contribute to release by breakage of bonds between cellulose fiber, starch and mineral fillers. Starch is the third most important paper furnish being the most significant components cellulose fiber and clay the first two. Starch is added to the paper in various forms for different purpose. For example cationic starch is added as a retention aid for mineral fillers. When cationic starch is adsorbed on the cellulose fiber, the adsorption is irreversible. Over 85% of the adsorbed cationic starch remains on the fiber in hot water; and only redissolves under acid conditions. Therefore, acid pulping may promote the breakage of bonds between cellulose fibers, fillers and starch and thus release more mineral fillers particles for flotation.
The removal of mineral fillers by flotation is not very satisfactory at alkaline conditions, This is a well known fact in de-inking plants. It is the usual practice in recycle mills to have a washing stage after alkaline flotation in order to remove residual mineral fillers from the furnish.
In the case of acid conditions, as found in this research program, the enhanced removal of mineral fillers can be related to the reversibility of the mineral filler surface charge with respect to pH. Anatase has a PZC that varies between pH values of 4.7 and 7.0 depending upon the mineral source and method of preparation. The same is true for kaolinite in which case the PZC values varies from 4.0 to 5.0 depending upon the mineral source and method of preparation.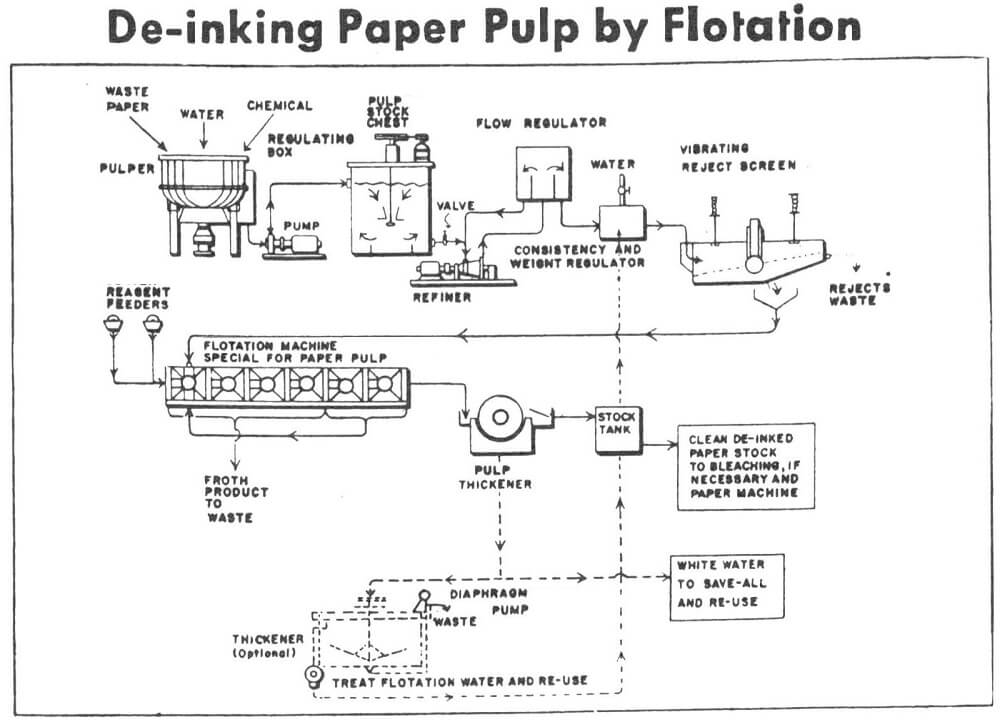
The optimum flotation pH of 6.3 for mineral fillers may be related to collector (fatty acid) adsorption. Drelich and others have noted that oleate adsorbs at the titanium dioxide surface. However, the adsorption is only significant at pH values of 7.0 to 7.5. From our de-inking flotation results, the removal of anatase starts to drop at pH values of less than pH 6.3. It is probable that other factors, as well, account for the pH dependence of mineral fillers during de-inking flotation.
The success of flotation of toner particles and mineral fillers under acid flotation conditions might also be related to the stability of the froth. It was observed experimentally that for acid flotation conditions the froth stability increases considerably compared to the froth behavior for alkaline flotation conditions.
According to the results presented, de-inking flotation of laser printed waste is more effective if it is operated under acid pulping conditions because the overall enhancement in the removal of toner and mineral filler particles. Another advantage of acid pulping and flotation is to reduce the chemical cost and the treatment cost of the water circuit due to low COD (chemical oxygen demand) and BOD (biological oxygen demand) levels.
It appears the caustic pulping reduces the abrasion action and/or cause fiber swelling both of which result in the release of larger toner particle size.
Independent of the pulping conditions, the flotation de-inking response is strongly effected by changes in pH. It was showed that the removal of both toner and mineral fillers is significantly improved under acid flotation conditions as compared to the results for conventional alkaline flotation de-inking.
Good flotation of both toner and mineral fillers from MOW can be achieved under the same conditions at pH 6.3 for both acid and alkaline pulping conditions. However the results are significantly improved for the acid pulping case.