Table of Contents
 A Flocculant like the Dry polymer (Percol 156) is used throughout the process to flocculate fine particles and increase the solids settling, filtration and clarification rates.
A Flocculant like the Dry polymer (Percol 156) is used throughout the process to flocculate fine particles and increase the solids settling, filtration and clarification rates.
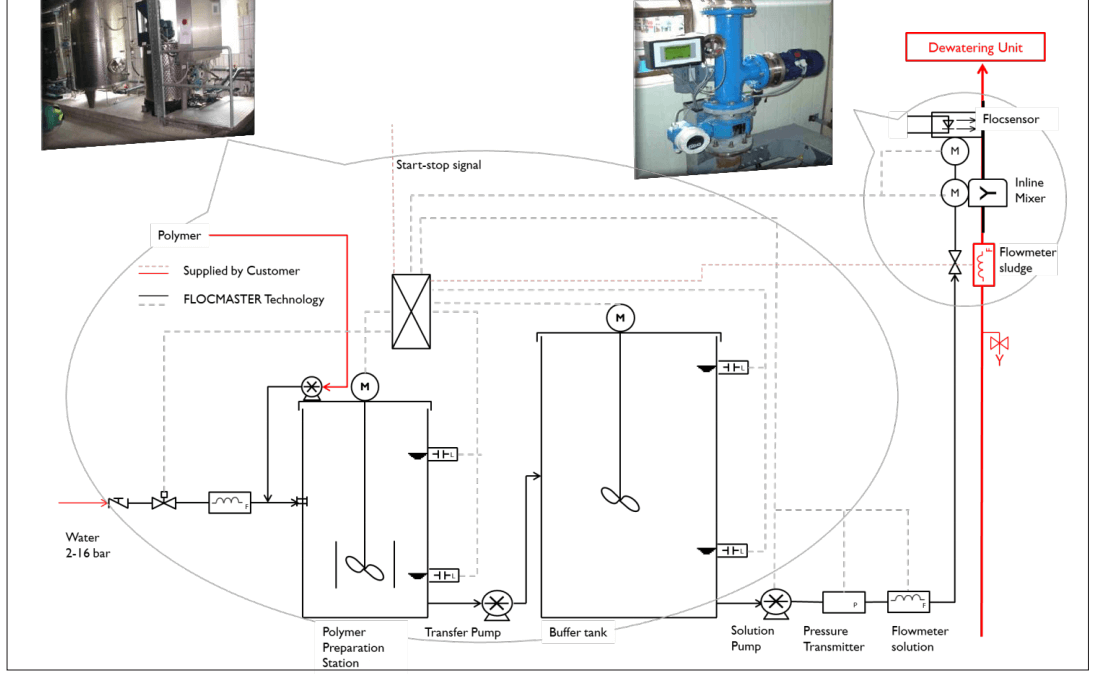
Flocculant is prepared and aged in the skid mounted automatic flocculant mixing system. A control panel for the system is mounted on the same skid. Refer to the equipment manufacturer’s operating manual for detailed operating instructions and calibration.
Wetted and aged flocculant from the mix system is transferred to a 2.75 meter diameter x 2.75 meter high flocculant mix tank on an automatic regular basis at a rate of 1200 litres per batch, at a concentration of 0.5%, where it is further mixed and aged. The mix tank is equipped with a low level switch and a high level switch, which provide signals for the mix system and will also alarm on low and high levels at the mill control panel annunciator. A locally operated 2 HP agitator in the mix tank will ensure that the flocculant will maintain complete solubility.
After the desired aging/mixing period in the flocculant mix tank, the flocculant is transferred, with a 10 HP 5″ x 4″ progressive cavity pump to the flocculant holding tank or to the flocculant day tank. The flocculant day tank is equipped with a level gauge glass and overflows back to the flocculant holding tank. The flocculant holding tank is equipped with a pressure gauge, indicating relative solution level in the holding tank, and a high level switch, which will alarm on a high level condition.
Flocculant is fed to the clarifiers and the Argentite drum filters from the flocculant day tank with metering pumps. Flocculant is fed to the thickener and drum filters from the flocculant holding tank with metering pumps. The operation of the flocculant metering pumps is identical to those for the other reagents.
As mentioned, the flocculant prepared in the flocculant system has a concentration of 0.5% w/w. Immediately downstream from the flocculant metering pumps, the flocculant must be diluted with fresh water to a final concentration of 0.05% w/w, i.e., for every litre of 0.5% flocculant solution, 10 litres of water are required to dilute it to a 0.05% flocculant solution.
Each fresh water dilution line has a flowmeter and globe valve which must be manually adjusted to give the proper dilution.
Preparing Flotation Reagents and a Lime Addition System
Measuring Flocculant Strength
The determination of floc strength is important in order to evaluate the ability of flocs to withstand the turbulent forces that would be encountered either during filtration or transportation of flocculated mass to settling ponds. Previously reported studies, have used several different techniques to evaluate floc shear strength. A general criteria used in all these studies, was based on the evaluation of size and number of flocs that result when a parent floc is subjected to a known shear stress. Hannah et al. (1969), measured the number of flocs that would be generated in a particular size range, when a parent floc is drawn through a 70 µm orifice under a known pressure gradient. However, this study was only qualitative and the density of flocs, which was shown to vary with the concentration of inorganic electrolyte used, was not considered. Tambo and Hozumi (1979), devised a special flocculator to measure the strength of flocs obtained by coagulation of kaolinite with aluminum sulfate. The energy dissipated in the system was calculated from the torque measured at a given speed of agitation and the floc strength was evaluated using the maximum diameter of the flocs obtained and its corresponding density. Glasgow and Hsu (1982) made a photographic observation of the disintegration of individual flocs upon interaction with a turbulent jet, and measured the resulting size and number of flocs produced for a given energy input. A common observation that was made in the later two studies, is that strength of a floc decreases with increasing floc diameter. This is in agreement with the fact that floc density varies inversely with its size. The flocculant dosage and pH also had a significant effect on strength of flocs obtained. Organic polymers were found to produce flocs which were more than twice as strong as those produced by inorganic electrolytes.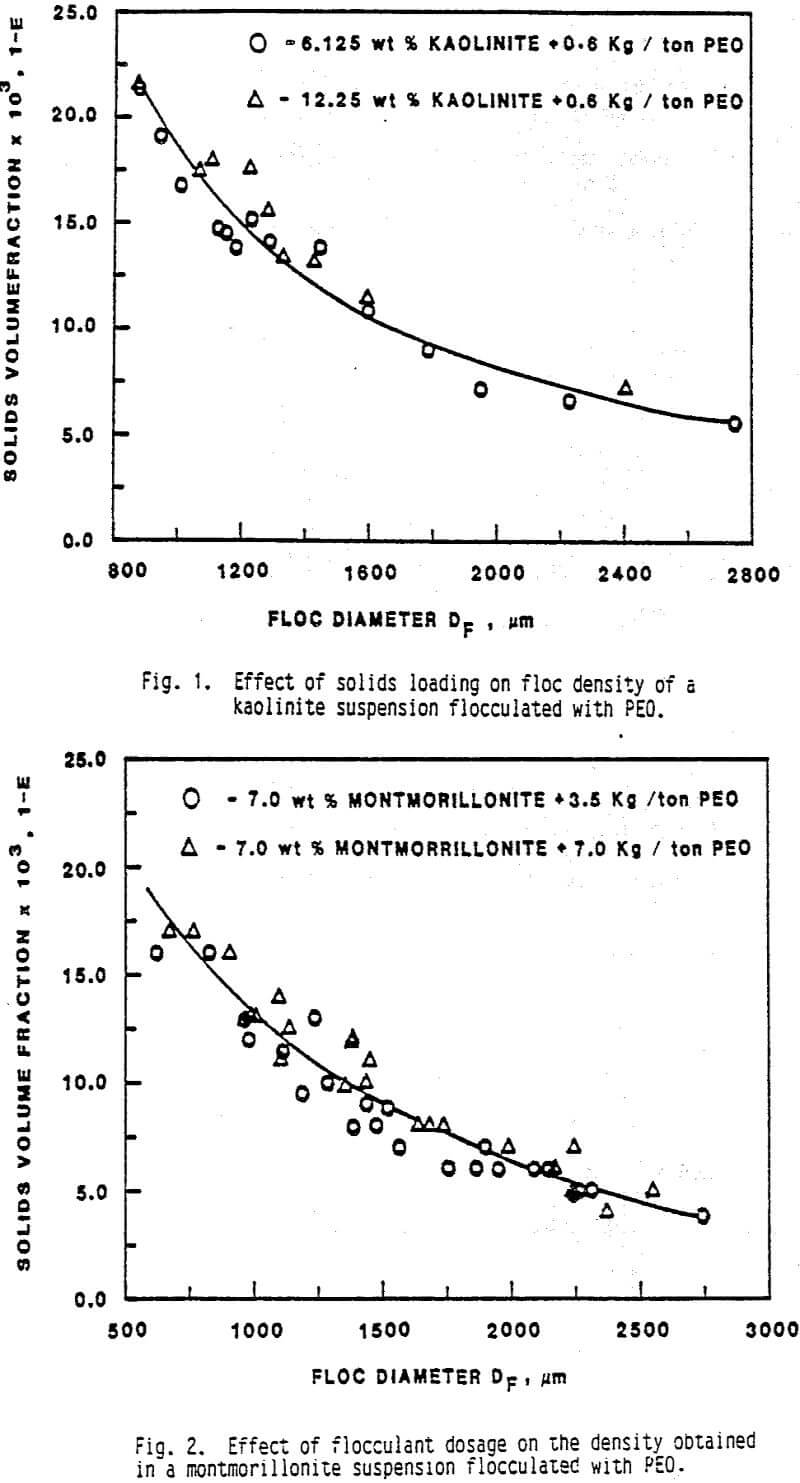
In all the above studies strength of flocs were measured under viscous flow conditions, as it was argued that typical coagulation process is carried out under those conditions. However, as mentioned earlier, it would be more appropriate to determine the floc strength under turbulent conditions that would be experienced either during filtration or transportation of the flocculated mass to settling ponds e.g. by pumping. Experiments are currently being carried out in our laboratories, to determine the strength of flocs under turbulent conditions of operation. A fully baffled cylindrical mixing tank provided with an impeller of standard design, is used to create the required shear field. The energy dissipated is calculated using the input energy to the stirrer and size and density distribution of flocs are measured using the procedure described earlier. A theoretical model is being developed to evaluate the binding energy from the experimental data and to correlate the floc strength with adsorption characteristics of the polymer on a given mineral. These results will be reported later.
Effect of agitation intensity
It has been reported that increase in intensity of agitation results in higher densities of larger flocs. An increase in agitation intensity would result in increased collision frequency between particles/aggregates. However, as discussed in earlier section, this would not lead to an increase in floc density. It is generally known that the density of flocs not only vary with their size, but there will be a distribution of densities even for flocs of the same size. An increase in agitation intensity would thus lead to preferential break-up of weaker flocs of a particular size, resulting in a distribution with a higher mean density. Large flocs rupture more easily than smaller ones, both due to higher porosity and the larger stresses they are subjected to in a turbulent field. Thus the effect of agitation intensity on floc density is encountered only in larger floc size ranges.
Effect of flocculant dosage
The results of the experiments conducted to evaluate the effect of polymer dosage on densities of montmorillonite flocs are shown in Figure 2. It is indicated that, in the range studied, the flocculant dosage has no significant effect on floc densities achieved. This observation is in agreement with the results reported. It is known that, below the point of optimum surface coverage, an increase in flocculant dosage would result in higher collision/sticking efficiency between polymer coated particles/aggregates and therefore in a lower floc density. Also the flocs formed at a higher flocculant dosage are expected to be stronger, due to more attachments between particles resulting from a higher adsorption. This would lead to less floc rupture at a given agitation intensity at the higher flocculant dosage thereby yielding a distribution with a lower mean density. This trend was observed in a study of coagulation of kaolinite with alum. However, both in the present study and the one carried out, it is possible that the range of flocculant dosages examined were not wide enough to observe the expected changes in floc density.