Table of Contents
It is often impracticable to apply the ordinary parting assay to the examination of low-standard alloys of gold with other metals. These are then tested by various other methods, of which a summary is given below, the alloys being grouped in four series for convenience:
A. Alloys requiring scorification.
B. Amalgams.
C. Alloys containing members of the platinum group.
D. Tellurium compounds.
Scorification of Alloys

Alloys of arsenic or antimony are reduced to a fine powder and scorified with thirty parts of lead and a half part of borax. If the slag becomes pasty towards the end of the operation more borax is added, a little at a time. If the lead button obtained is hard, a second scorification is necessary, with the addition of more lead. There is some loss of gold in the slag.
- Iron or Manganese Alloys
The operation is tedious and difficult with these alloys, as they are difficult to fuse, having higher melting points than pure gold, and the oxides of iron do not form easily fusible compounds with the litharge. An extremely high temperature is required; ten parts of lead, one of borax, and one of silica usually suffice.
- Cobalt and Nickel
Twenty parts of lead are used, but no borax at first, so that the oxidation of the nickel may not be hindered. A very high temperature and the subsequent addition of two parts of borax are necessary. Several successive scorifications are required as nickel and cobalt are difficult to oxidise. - Zinc
Oxide of zinc does not form a fusible mixture with litharge, and the slag is only rendered pasty by borax, unless it is added in large quantities. Gold is lost in the slag, but the loss is minimised by slagging off the zinc as rapidly as possible. Use fifteen to twenty parts of lead and two to three parts of silica, with a little borax. - Tin
Twenty parts of lead are required ; oxide of tin is rapidly formed, but the slag is not easily fusible. Large amounts of borax are necessary, or still better, borax mixed with potash which forms a fusible stannate with SnO2. - Aluminium
Alloys containing this metal cannot be assayed by scorification and cupellation. As soon as fusion takes place in the muffle, aluminium floats to the top of the bath, being of low density, and is rapidly oxidised, producing alumina which forms an exceedingly infusible scoria not easily removed by litharge. The production of the latter, moreover, is checked by the scum.
Mr. Edward Matthey observes that the removal of aluminium by digestion in hydrochloric acid, and collection of the residual gold, does not yield satisfactorily results. The process he recommends is as follows:
Accurately weighed portions of 50 grains each of the alloys are fused with litharge, under a flux of potassium carbonate and borax with a small proportion of powdered charcoal, and the resulting slag re-fused with a further small quantity of litharge and powdered charcoal. The lead buttons containing all the gold (the aluminium having combined with the fluxes employed) are cupelled, and the resulting gold cupelled with silver and parted with nitric acid in the usual manner. The assays must be worked with checks or standards of fine gold and pure aluminium. In the majority of the preceding cases it is better to analyse the alloys by wet methods.
Fire Assaying of Amalgams
The alloy is placed in a weighed porcelain crucible and gradually heated so as to drive off the mercury. After the greater part of the mercury has been driven off the temperature is raised to a full red heat which is maintained for half an hour. About 0.1 per cent, of mercury still remains in the gold after this treatment and can only be completely removed by cupellation and parting. Checks must be used, as the loss of gold in the operation may amount to 1 per 1,000.
A better method is to dissolve the mercury in nitric acid mixed with an equal volume of water. A porcelain crucible or basin should be used and gentle heat applied. If the action is not too violent the gold does not break up, but is left as a spongy coherent mass. It is washed, first with nitric acid, and finally with water, and ignited. Mercury may be made use of to collect very finely-divided precipitated or parted gold into a coherent mass, the mercury being removed by nitric acid.
Fire Assaying Platinum Group Elements
Cupellation must be performed at a higher temperature than usual, and the iridescent bands are seen to remain longer, although they are less numerous.
Platinum Gold Alloys Fire Assaying
The button obtained by cupellation is dull and crystalline. If the alloy contains as much as 7 or 8 per cent, of platinum the cupellation proceeds slowly, brightening is only obtained at a very high temperature and the button appears flattened, and has a rough crystalline surface and a grey colour. If more than 10 per cent, is present, brightening does not occur at all, and the other features just mentioned are more strikingly exhibited. On parting, the platinum is partly dissolved with the silver, but the assay-piece must be boiled in acid for a long time, and the parting is incomplete. A second and even a third parting may be necessary. Unless proofs are made up of similar composition the results are not satisfactory if more than 1 or 2 parts of platinum are present per 1,000 of alloy.
According to results obtained in the laboratory at the Royal Mint by Prof. Stansfield, it is impossible to free buttons containing platinum from lead at the ordinary temperatures attainable in a muffle. It is necessary to employ the oxy- hydrogen blowpipe for the purpose, with the result that the losses are irregular. In the cupellation of pure platinum, buttons weighing from 0.002 to 0.01 gramme retain about 10 per cent, of their weight of lead, and those weighing from 0.04 to 2 grammes retain about 33 per cent. f parting is effected by boiling in concentrated sulphuric acid instead of in nitric acid, almost all the platinum remains undissolved with the gold. Chaudet recommends the following proportions of silver for this purpose:
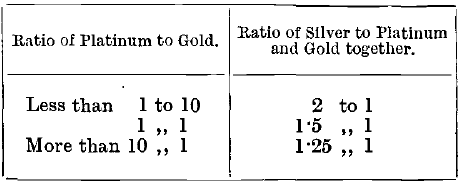
The reason for the reduction, shown in the table, in the amount of silver when the platinum is increased is that, according to Chaudet and Haindl, considerable quantities of platinum are dissolved, and the error caused this is corrected by leaving some silver undissolved in the cornet. The losses of platinum, however, are very irregular, and the method is not a good one. The reason for the variation is probably the lack of homogeneity in the buttons and prills, as gold-platinum alloys show a considerable amount of liquation.
The platinum is not dissolved by nitric acid if zinc or cadmium is substituted for silver.
Mr. Edward Matthey recommends the following method for alloys of platinum 900, and gold 100 parts :—50 grains each are taken of the alloys and treated with an excess of nitrohydrochloric acid which gradually dissolves the whole. The resulting solutions of platinum-gold chloride are then evaporated nearly to dryness to drive off the free acid and diluted with distilled water to about 20 c.c., a degree of strength ascertained by experiment to be the best for the precipitation of the gold. The metallic gold is thrown down by means of crystals of oxalic acid, and is carefully washed, dried, and weighed. It is necessary to use checks.
The estimation of platinum in its alloys with gold and silver has also been studied by M. Forest, assayer at the French Mint.
Fire Assaying Palladium-Gold Alloys
The palladium is dissolved in parting if the weight of silver is at least three times that of the gold, yielding an orange coloured solution. Matthey recommends double parting. Separation may also be affected by fusion with six to eight parts of potassium bisulphate, and dissolving out the dark brown palladious sulphate by boiling water. A second fusion is usually necessary to render the gold residue quite pure.
As in the case of platinum, palladium may be separated from gold by dissolving the alloy in aqua regia, evaporating to dryness, taking up with water and precipitating the gold by oxalic acid from a very dilute solution : the palladium remains in solution.
Fire Assaying Rhodium and Iridium-Gold Alloys
Iridium, if present, always sinks to the bottom of the cupelled button as it is very dense (specific gravity = 21.38), and is not usually fused at the temperature of the muffle, but occurs in the state of fine black crystalline particles. Hence, when the button is rolled into a cornet with the lower face outwards iridium occurs as black sooty spots or streaks which are seen by a lens to fill up depressions in the surface of the gold.
Both rhodium and iridium are almost insoluble in aqua regia. If gold alloys containing both of them are parted in the ordinary way with nitric acid, only a small quantity of rhodium goes into solution with the silver. The residue consisting of the gold, the iridium and most of the rhodium may then be attacked by dilute aqua regia when the gold is dissolved together with only traces of the other metals. These may be separated by evaporating the solution to dryness and heating to dull redness, when the reduced metals being no longer alloyed may be completely separated by dissolving the gold in aqua regia. Iridium may be separated from gold by fusing it with fluxes, the charge being made up as follows:
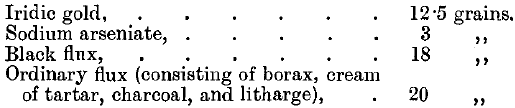
The iridium forms a speiss with the iron and the arsenic, and the lead button formed at the bottom of the fused mass contains all the gold. The estimation of osmium, iridium, and ruthenium in gold bullion has also been studied by Riche, Leidie, and Quennessen, assayers at the French Mint.
Assaying Tellurium Compounds
These must be treated by wet methods. The alloy may be dissolved in nitrohydrochloric acid, and the solution containing both gold and tellurium evaporated with a large excess of hydrochloric acid until no more chlorine is given off, when both gold and tellurium are readily precipitated by a current of sulphur dioxide gas. On attacking the precipitate with dilute nitric acid the tellurium is dissolved in the state of tellurous acid, and the gold residue may be dried and weighed, and its purity ascertained by inquartation and parting. The greater part of the tellurium may be removed from gold-tellurium alloys by boiling in nitric acid, and the residue can be cupelled and parted with very little loss of gold.
Wet Assay Methods of Gold Alloys & Compounds
Assays or complete analyses of gold bullion, natural minerals, can be made by the ordinary chemical methods given by Fresenius, Crookes, and others. From 1 to 5 grammes of bullion are usually enough, but a much larger amount is necessary if the alloy is nearly pure gold. M. Forest takes 300 grammes of gold bullion when examining it for small quantities of impurities. In general the residue left after prolonged action of nitric or sulphuric acid is not sufficiently pure to weigh as gold, and complete solution in aqua regia is usually necessary. From the solution the gold may be precipitated by:
- (a) ferrous sulphate,
- (b) sulphurous acid,
- (c) oxalic acid,
- (d) sulphuretted hydrogen,
- (e) ammonic sulphide, followed by the addition of hydrochloric acid.
The following remarks may be of value in aiding the chemist in his choice of a precipitant in any particular case. Nitric acid must always be expelled from the solution by warming with successive additions of hydrochloric acid. The acid solution must not be heated too strongly or loss of gold chloride by volatilisation occurs. Some other chlorides escape more freely. Ferrous sulphate and sulphurous acid act well in strongly acid (HCl) solutions ; oxalic acid, sulphuretted hydrogen, and ammonic sulphide best in presence of small quantities of HCl. The solution should be dilute (say 1 part of gold in 300 of water), so that other metals may not be carried down by the gold. Sulphate of iron gives a very finely-divided precipitate which is difficult to wash by decantation without loss ; precipitation is slow in cold solutions. Oxalic acid causes plates and scales to form which are readily washed and are very pure; it acts best in boiling liquids, but a temperature of 80° for forty- eight hours suffices; in the cold or in the presence of much hydrochloric acid or alkaline chlorides the action is very slow and partial; a large excess of the precipitant must be present. Oxalic acid is used for solutions containing metals of the platinum group, which are not precipitated by it. Alkaline oxalates act better than the free acid.
Sulphurous acid is an excellent precipitant for most solutions. It acts rapidly and completely in the cold, and does not readily precipitate other metals, except tellurium. Sulphuretted hydrogen is used in the absence of all other metals whose sulphides are insoluble in hydrochloric acid.
In all cases careful consideration must be given to the nature of the base metals present, and the precipitant which will not render any of them insoluble must be selected.
