Table of Contents
This investigation has shown that lead and zinc sulfide minerals, in pulps containing particles all less than 9 microns in size, may be selectively recovered by froth flotation. All but one of the samples yielded lead concentrates-containing at least 57 percent Pb. Selective recovery of lead in the lead concentrate from extremely fine-grained ores was improved by use of a chemical dye (diphenylthiocarbazone) as a galena promoter.
Data on the cost of grinding to sizes finer than 400-mesh are scarce. Some projection of costs, however, may be made by considering such factors as ore hardness, feed size, pulp density, product size, and quantity treated relative to the power and steel requirements reported by commercial lead-zinc operations. On this basis, it is estimated that the costs of fine grinding (as described in these tests) might be three to five times greater than current expenditures for commercial grinding through 100-mesh.
An estimate of flotation reagent costs, based on chemical similarity to standard collectors and quantities used in testing, indicates that the use of dithizone might double the reagent expense.
Such an analysis indicates that combined application of extremely fine grinding and use of dithizone would possibly increase current average milling costs 100 percent. The improved metallurgy and increased utilization of reserves might, however, show an overall economic advantage.
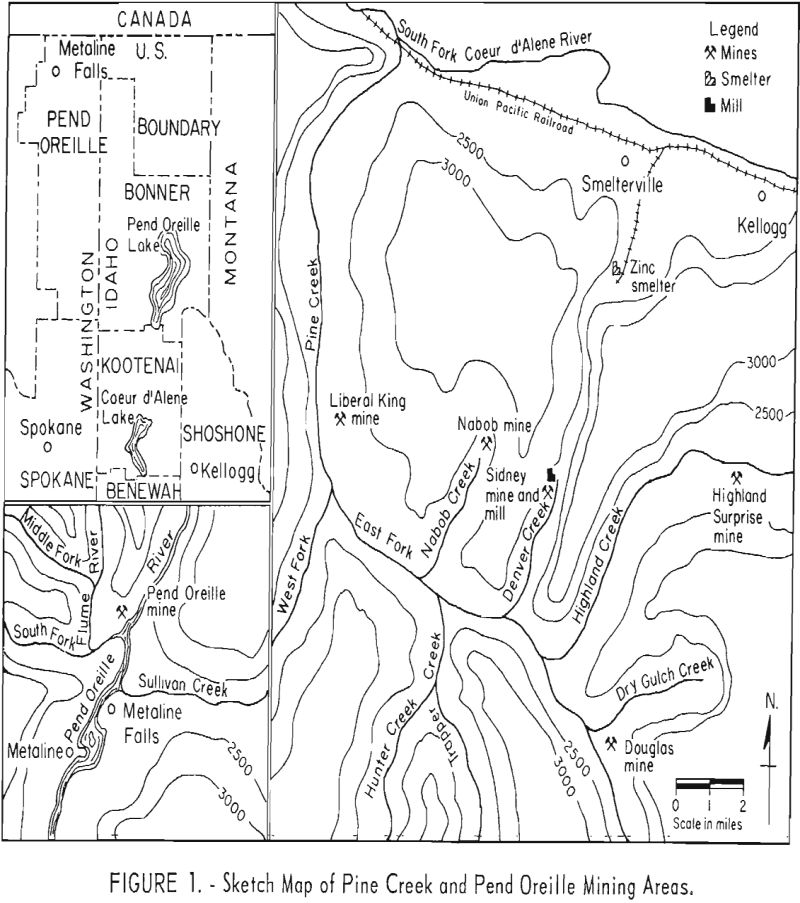
This report summarizes the results of flotation studies conducted by the Federal Bureau of Mines on samples of extremely fine-grained lead-zinc sulfide ores. Samples from five ore deposits in the Pine Creek area, Coeur d’Alene district, Idaho, and one deposit in the Metaline district, Washington, were studied at the Albany Metallurgy Research Center to determine the recovery of sulfide lead and zinc by flotation.
During the investigation it was found that flotation could be effectively applied to the selective recovery of exceedingly fine-grained lead and zinc sulfide minerals. Separation of galena and sphalerite was obtained on pulps ground through 1,600-mesh, in which most of the particles were smaller than 5 microns. Selectivity and recovery of minerals in this fine size range were enhanced by use of a chemical dye, diphenylthiocarbazone, as a galena promoter. Although unsuccessful on one ore, application of the dye in treatment of two other very fine-grained ores yielded lead concentrates containing over 62 percent Pb at recoveries in excess of 85 percent. More than 55 percent of the lead in these concentrates occurred as particles smaller than 2 microns.
Overall results from treatment of six ores by fine grinding and flotation included lead concentrates ranging in grade from 57 to 65 percent Pb with 72 to 95 percent recovery and zinc concentrates varying from 52 to 63 percent zinc at recoveries of 81 to 93 percent. A seventh ore did not respond to selective concentration of the sulfide minerals. Studies on mill feed samples indicated generally that by grinding to approximately twice the fineness of the commercial flotation feeds, lead and zinc recovery could be improved from 5 to 8 percent in their respective concentrates. In like manner, the zinc-concentrate grade could also be increased by about 9 percent with a reduction in lead content of approximately 7 percent. Though test data demonstrated that mill results could be improved by finer grinding, the grinding necessary for particle liberation on some ores was considered economically impractical with present techniques.
Statistics on production of lead-zinc ores in the United States indicate that the mining districts of Coeur d’Alene, Idaho, and Metaline, Wash., are two principal sources of these ores. In the 10-year period beginning with 1948, the combined output of the two districts accounted for about 21 percent of the lead and 13 percent of the zinc recovered from domestic lead-zinc mining. Unpublished data on measured and indicated ore reserves show that the two districts also contain a significant part of the Nation’s lead-zinc resources. The solution of problems related to the beneficiation of these ores is thus of considerable economic importance to the Northwest and the Nation.
Typical of the complex sulfide ores often encountered in these districts are those of the Pine Creek area of the Coeur d’Alene district and the Pend Orielle mine of the Metaline district. These ores, consisting of disseminated mixtures of coarse-to-fine-grained sulfides, present problems in the selective concentration of sulfide minerals, particularly in the securing of a high quality lead concentrate. Information obtained on the milling of Pine Creek ores showed lead concentrates analyzing as high as 26 percent zinc and zinc concentrates containing up to 17 percent of the total lead. The interlocking of sulfide lead-zinc minerals at fine sizes and the loss of slimed particles during flotation were indicated as the two principal deterrents to selective recovery. Milling practice at the Pend Oreille mine showed high recovery of lead and zinc with a lead concentrate grade averaging over 70 percent lead. The zinc concentrate grade of 55 to 56 percent zinc was considered low, however, by the company. The low grade of the zinc concentrate was determined to be the result of intimate association of carbonaceous matter in sphalerite.
According to Taggart, particles treated in commercial flotation pulps have ranged from one-eighth inch to colloidal size. A review of 20 operating lead-zinc mills indicated that the current practical lower limit in flotation involves treatment of pulps wherein no more than about 25 percent of the particles are less than 800-mesh (20 microns). Gaudin, Groh, and Henderson in a discussion of operating plants, pointed out the generally reduced mineral recovery with diminishing particle size. De Bruyn and Modi, using as a basis an investigation with quartz, gave empirical evidence indicating an optimum particle size range for flotation of 400-to 1,600-mesh. More recently, Frommer and Fine reported successful treatment of slimes (mostly minus 400-mesh) in fine tailings from a Missouri lead sulfide concentrator.
The Ores
Laboratory beneficiation research involved treatment of seven samples representative of six lead-zinc deposits or portions of them. Six of the ores originated from the Nabob, Highland Surprise, Sidney, Liberal King, and Douglas mines in the Pine Creek area of the Coeur d’Alene district of northern Idaho. The seventh ore came from the Pend Oreille mine, Metaline district, northeastern Washington. A sketch map, given In figure 1, illustrates the geographical relationship of the different operations.
Physical Character
The lead-zinc sulfide ores of the Pine Creek area occur as replacement deposits in fissure veins or fillings in a siliceous host rock. They are generally fine-grained aggregates of sphalerite, galena, and pyrite associated with lesser amounts of pyrrhotite, marmatite, or chalcopyrite and varying quantities of feldspar, ankerite, siderite, or sericite in a quartz matrix. There is usually two to three times as much zinc as lead. Sulfide minerals and siliceous gangue are intimately associated; locking with gangue occurs frequently in the minus 400-mesh range. Although much of the sphalerite and galena are separated at 200-mesh, galena is often present as inclusions as fine as 1 micron in the sphalerite.
Ore from the Pend Oreille mine, Metaline district, Wash., which also occurs as a replacement deposit, appears in minor breaks, breccias, or cavities within a limestone horizon. It is somewhat coarse-grained and composed generally of sphalerite, pyrite, and galena in a predominantly quartz-dolomite matrix. Two significant differences from the Pine Creek ores are the higher ratio of sphalerite to galena (about 10 to 1 compared to 2 or 3 to 1 in the Pine Creek ores) and the presence of carbonaceous material distributed throughout much of the sphalerite. The lead and zinc minerals are locked to about 150-mesh, whereas the carbonaceous inclusions in the zinc sulfide mineral range from 5 to 20 microns.
Chemical Description
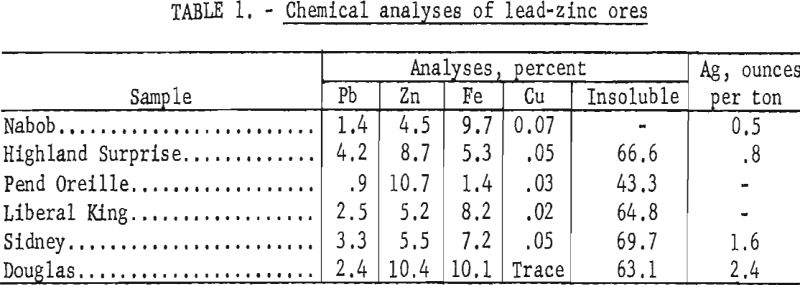
Chemical analyses of representative portions of each ore sample are shown in table 1.
Laboratory Studies
Petrographic examinations of the ore samples and the products from preliminary tests showed that interlocking of fine-grained sulfides was essentially the cause of contamination of concentrates by other sulfide minerals. Although most of the sulfides were liberated from gangue minerals at a relatively coarse grind, considerable interlocking of sulfides occurred in the subsieve range. Consequently, though operating-mill recovery of the total base metals was generally good, ranging from 85 to 98 percent, concentrate grades were not always satisfactory. With the exception of one ore, the laboratory investigation attempted to improve the separation of the lead and zinc minerals commensurate with high recovery of the minerals in the respective concentrates.
Whenever practical, treatment of an ore sample followed conditions simulating actual mill practice. Modifications of the mill scheme included generally finer grinding and change of reagents. On some samples, however, the intimate mineral associations required grinding to a particle size beyond practical limitations. Ultrafine grinding and flotation were applied to three ores primarily to determine the response to flotation of extremely fine particles, and to demonstrate selective separation of sulfide minerals in the minue 20-micron range.
Though recovery of sphalerite in the zinc concentrate varied according to the amount of that mineral going to the lead concentrate, in general, no special problems arose regarding its flotation. As an example, zinc concentrates of 57 to 61 percent Zn were obtained on finely ground samples of the Liberal King and Douglas ores. Accordingly, much of the testing was restricted to flotation of galena, during which phase the application of more selective promoting agents was emphasized.
Conventional laboratory equipment was used in the tests except for a small regrind mill suitable for batch treating 100- to 300-gram quantities. The 4-inch-diameter by 6-inch-long cylindrical steel mill was designed with lifters and operated with equal portions of ¼-, ½-, and ¾-inch balls. Mineral particle size and distribution in the subsieve range were determined with a petrographic microscope and net-micrometer eyepiece attachment. All samples used were collected by Bureau of Mines engineers.
Nabob Ore
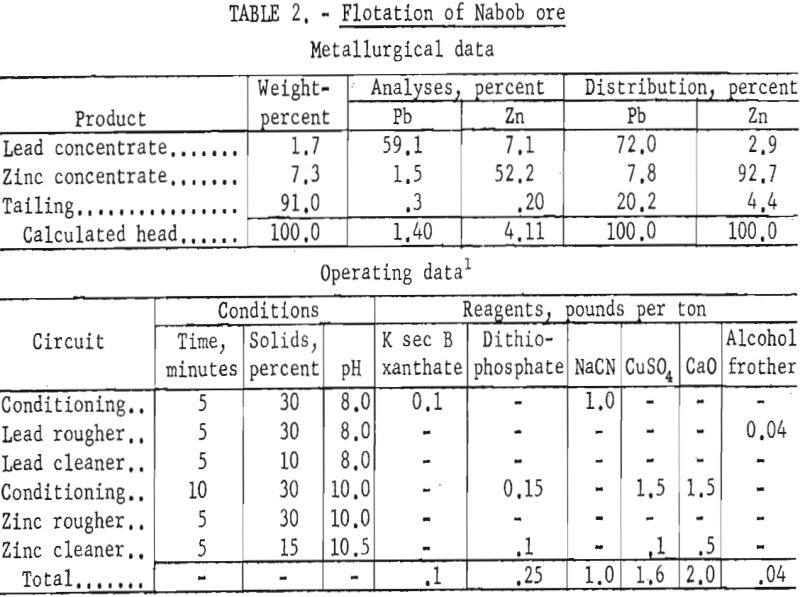
A small sample of the Nabob Company mill feed was received for beneficiation study. Chemical analyses indicated that the sample contained less than half the lead content of the average reported mill heads.
Petrographic examination of the sample and mill products indicated that grinding finer than 65-mesh (approximate mill grind) was necessary for liberation of minerals. Locking of lead and zinc sulfides with pyrrhotite and siliceous and argillaceous gangue appeared to be the main cause of low lead recovery, and interlocking of galena with sphalerite impaired selective separation of the lead from zinc.
Though the mill reported lead concentrate grades of 70 percent, lead recoveries were about 64 percent in the lead concentrate and 25 percent in the zinc concentrate. Zinc recovery in the zinc concentrate was about 82 percent and the concentrate grade was reported as about 38 percent Zn.
Laboratory testing showed that the mill results could be improved by grinding the ore through 200-mesh and using a dithiophosphate collector for the zinc at a pulp pH of 9.5 to 10.5. Batch-test results showed lead concentrates of 59 to 66 percent Pb with recoveries of 72 to 66 percent. Zinc concentrate grades varied from 50 percent Zn at 96 percent recovery to 52 percent Zn at 93 percent recovery. Table 2 gives the results of the most simple but effective reagent scheme applied.
Highland Surprise Ore
This ore lot represented a composite of grab samples of the Highland Surprise mill heads taken during a 10-day period.
The sample contained, in addition to the usual quartz and sulfide constituents, some pyrrhotite, limonite, tetrahedrite, and calcite. Liberation of sulfides from gangue was nearly complete in the minus 200-mesh fraction. Intergrowths of galena and sphalerite, however, were as fine as 10 microns and resulted in as much as 15 percent of the zinc appearing in the lead concentrate.
Laboratory results showed that finer grinding increased the lead concentrate grade about 9 percent and decreased the zinc content about 3 percent below plant grades. In addition, the zinc concentrate grade and recovery were increased 3 and 10 percent, respectively, over commercial results. The mill grind was reported as 100 percent minus 65-mesh, whereas the most effective laboratory primary grind was 100 percent minus 150-mesh or 74 percent minus 325-mesh. Though no regrinding was conducted in the mill, improved metallurgy was obtained in the laboratory by regrinding the lead rougher concentrate to 95 percent minus 325-mesh. Conditions and results of a typical batch test are shown in table 3.
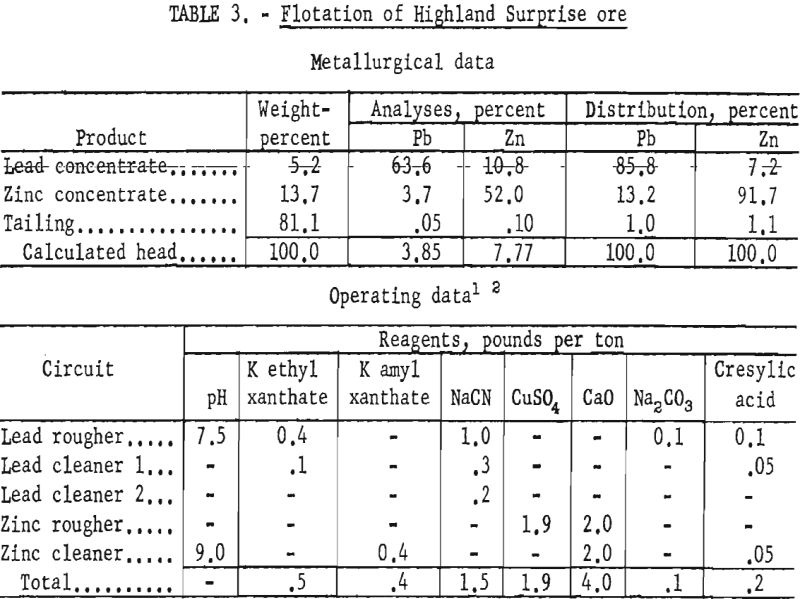
Based on the smelter schedules prevailing when the tests were made, but not considering the subsidy premium, the higher grade lead and zinc concentrates produced in the laboratory were calculated to have an increased value of $18.40 and $6.57 per ton, respectively.
Pend Oreille Ore
The sample submitted by the Pend Oreille Mines and Metals Co. represented a refractory ore present in various quantities in the daily mill feed. Mill results, particularly the grade of zinc concentrate, varied according to the amount of this material present. Although lead and zinc recoveries in their individual concentrates were reported as about 98 and 93 percent, respectively, the zinc concentrate grade usually was less than 56 percent Zn. A combination of smelter penalties and freight rates made it desirable for the company to produce a concentrate of plus 58 percent Zn.
Petrographic examination showed that the sample contained, in addition to quartz and dolomite, carbonaceous gangue associated almost exclusively with the sulfides. A study of sized fractions showed that the sphalerite and galena were essentially liberated in the minus 100- plus 150-mesh range. Much of the sphalerite, however, was locked with carbonaceous material at micron sizes. The smallest inclusions noted were approximately 1 micron.
Laboratory studies included fine grinding in conjunction with either selective flotation or depression of the carbonaceous material. The results of these tests demonstrated that a preliminary grind of about 82 percent minus 325-mesh satisfactorily liberated galena from gangue. Finer grinding to a particle-size distribution of about 98 percent minus 325 or 76 percent minus 1,600-mesh subsequently was needed to liberate sphalerite from carbonaceous gangue.
By comparison, mill primary grinding was carried out to approximately 100 percent minus 35-mesh or 50 percent minus 200-mesh whereas regrinding involved reduction to about 100 percent minus 65-mesh or 54 percent minus 325-mesh.
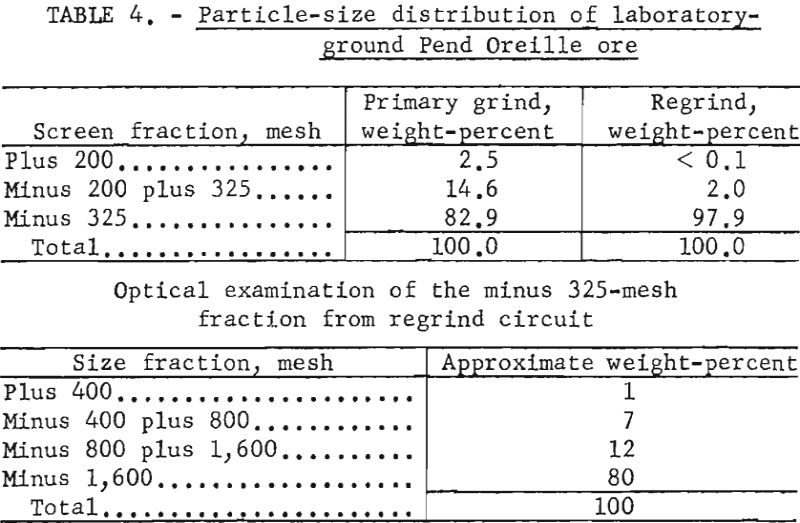
Size analyses were made on the discharge from the laboratory-test primary grinding circuit and from the regrind circuit. Table 4 shows the particle-size distribution of the coarser fractions as determined by screening and the size distribution of subsieve particles as determined by an optical micrometer.
Selective flotation of the carbonaceous matter was not satisfactory, as slimed galena and sphalerite were lost to the carbon product. On the other hand, attempts to depress carbon were relatively successful. Locked series tests indicated that, by use of a carbon depressant in the lead circuit, carbonaceous material was prevented from accumulating and diluting the lead or zinc concentrate. In batch-scale testing satisfactory results were obtained without a carbon depressant.
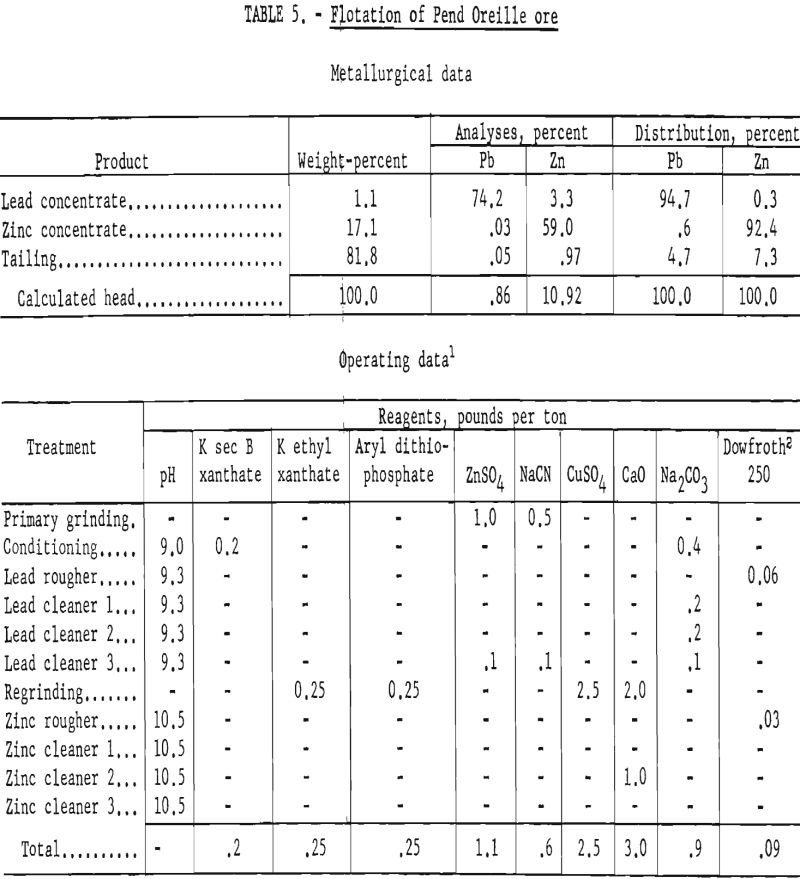
The operating data and results of a typical batch test are shown in table 5. All lead-circuit tail products went to the zinc circuit whereas zinc-circuit cleaner tails were combined with the rougher tails for analyses.
The company reported that modification of their mill circuit as suggested in the foregoing scheme gave results comparable to those shown in table 5. Although exact figures were not given, the cost of regrinding was reported to be lower than expected and was considered nominal in relation to the improved metallurgy.
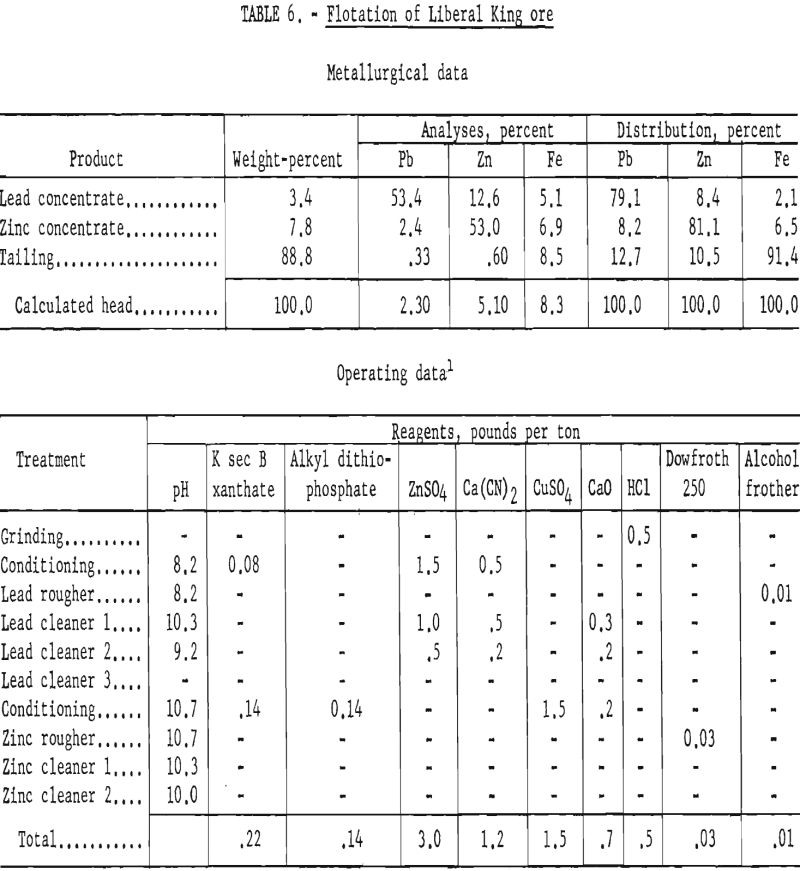
Liberal King Ore
This sample represented a fine-grained lead-zinc ore taken from the Liberal King mine operated by the Sunset Minerals Co.
Petrographic examination showed that the sample contained minor quantities of chalcopyrite, limonite, chlorite, and tourmaline in addition to the usual siliceous gangue. The sulfides (chalcopyrite, pyrite, galena, and sphalerite) were extremely fine-grained and intimately associated. Only partial liberation was noted in particles less than 200-mesh. Polished surface studies revealed galena inclusions as small as 3 microns within the sphalerite.
When the laboratory studies were made the company was concerned with two problems—lowering the zinc content of the lead concentrate and the iron content of the zinc concentrate. The lead product contained about 15 percent zinc, and the zinc concentrate contained 11 to 13 percent iron.
Laboratory studies showed that, by increasing the mill grind from 70 percent minus 200-mesh to 100 percent minus 400-mesh, the zinc in the lead concentrate could be reduced from an average of 15 percent to 12 percent. In like manner, the iron content of the zinc concentrate could be lowered from 12 percent to 7 percent.
Results of lead-zinc flotation on ore ground through 400-mesh are shown in table 6. Lead cleaner-tail products were sent to the zinc circuit, and the zinc cleaner tails were combined with the final flotation tails for analyses.
Since grade and recovery of lead were still not satisfactory, even at the finer grind of 400-mesh, a more detailed study was made to determine means of improving lead concentration. A microscopic examination of a lead concentrate revealed locking of galena with sphalerite in grains of minus 1,600-mesh, and some locking of both sulfide minerals with gangue was noted in grains of 800-mesh.
A series of fine-grinding and lead-flotation tests showed that primary grinding through 400-mesh and regrinding of the first lead cleaner concentrate through 1,600-mesh provided adequate mineral liberation. Petrographic examination of the concentrates produced showed that the relatively small amount of locking evident occurred primarily in the minus 1,600- plus 3,200-mesh range. Although a few sphalerite grains contained galena inclusions about 3 microns in diameter or had galena locked at the periphery, most of the sphalerite contained in the lead concentrate consisted of free grains from 1 to 2 microns in diameter. This fine-grained sphalerite tended to gather around the galena particles as a mechanical-locking phenomena when observed in water; this condition was not observed in index-of-refraction oils. Evaluation of data on high-grade lead concentrates from each of the Liberal King, Sidney, and Douglas ores indicated that at least 55 percent of the recovered galena occurred as particles smaller than 2 microns in size. Particle-size distribution analyses of products from a typical grind, as represented by a flotation concentrate and tailing from the Liberal King ore are shown in figure 2.
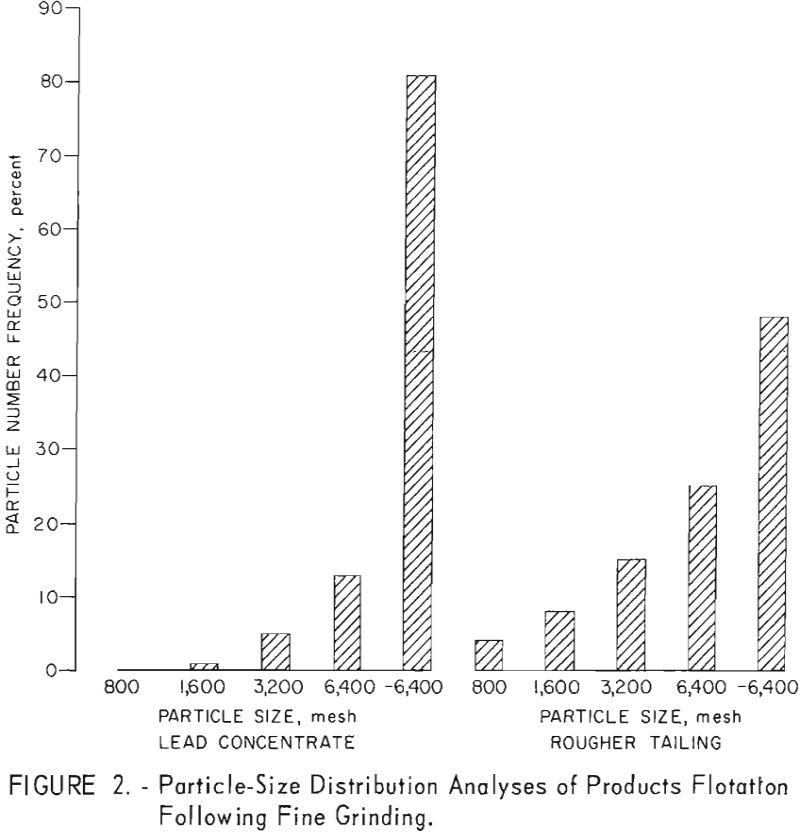
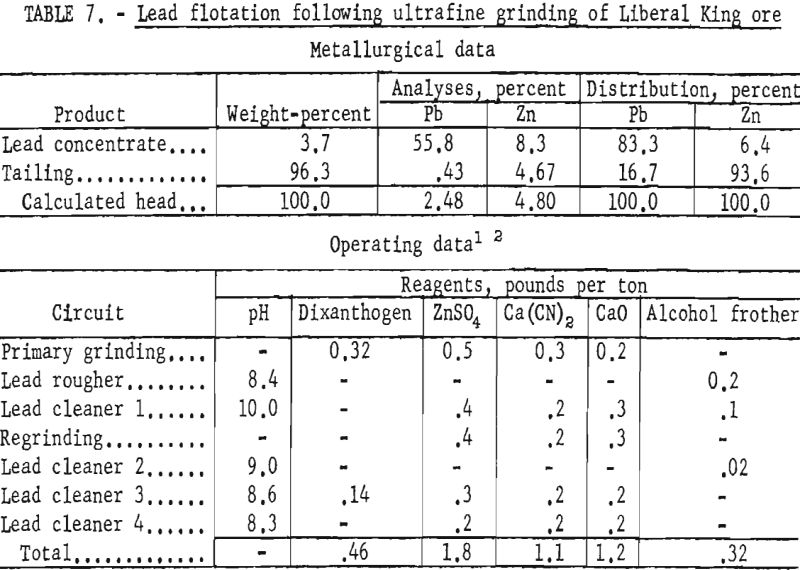
Test results showed that by regrinding the first lead cleaner flotation concentrate through 1,600-mesh the grade of lead and the lead recovery were increased about 2 and 4 percent, respectively. Zinc in the lead concentrate was also lowered from 15 to 8 percent. Results of ultrafine grinding on selective lead recovery are shown in table 7.
Since some fine sphalerite floated with the galena and some fine galena was lost to the flotation tailings when conventional collectors were used, another collector for galena was considered.
A chemical dye, diphenylthiocarbazone, was studied because of its similarity to known galena promoters and its reactive nature with the lead ion as evinced In analytical work. The dye, customarily referred to as dithizone, is a standard reagent used for detection of minute quantities of lead by col- orimetric and titrimetric methods. The structural formula for dithizone may be depicted as
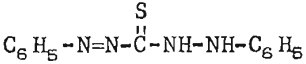
this is similar to thiocarbanilid, a common galena collector containing two less nitrogen atoms. The reagent was most effective when applied as a 1-percent solution in lime and water.
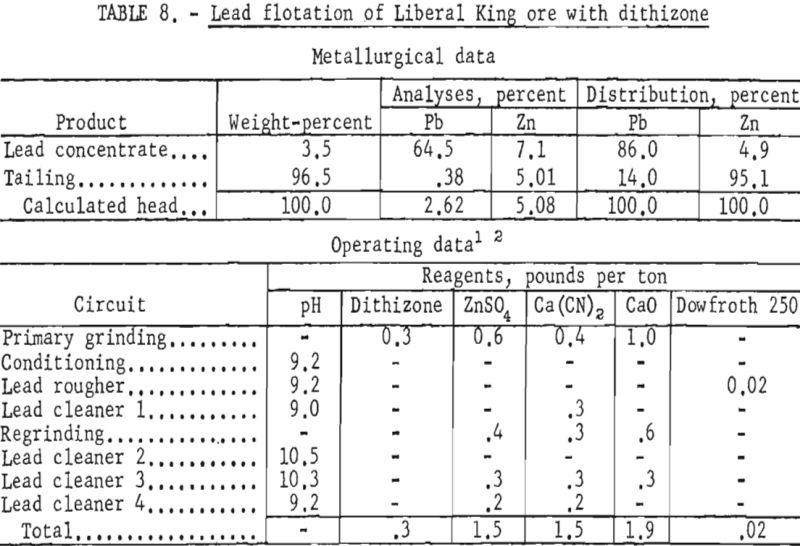
The evaluated data demonstrated that use of dithizone as a galena collector in minus 1,600-mesh pulps improved the lead content of the concentrate and the lead recovery about 9 and 3 percent, respectively. As shown in table 8, the zinc content of the lead concentrate was lowered slightly more than 1 percent.
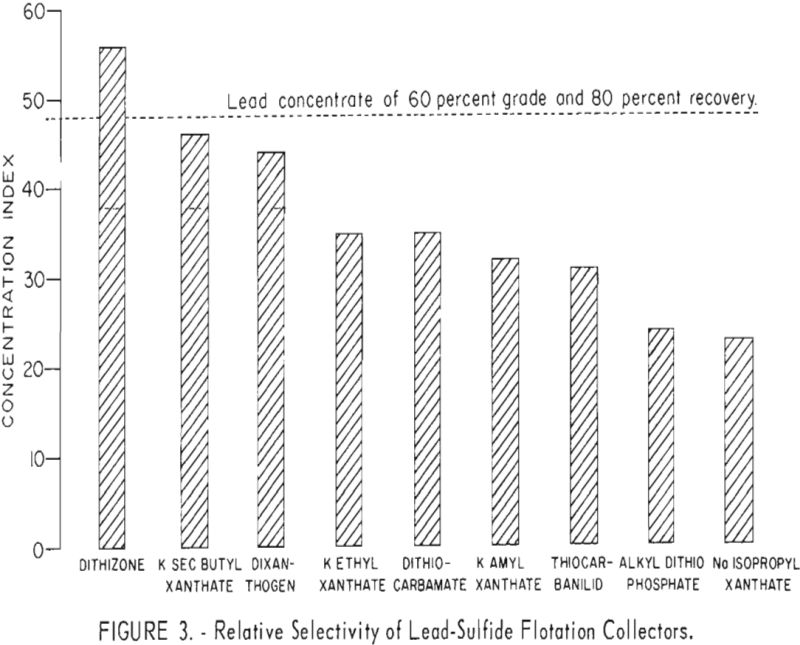
The effectiveness of dithizone in comparison with other galena-collecting agents is indicated in figure 3. For ease of illustration, a single number, referred to as the concentration index signifies the selective promoting ability of each reagent. The index was calculated by multiplying the percentage of the lead grade by the percentage of lead recovered in the lead concentrate and then dividing the result by 100. Indices calculated from results of duplicate tests with each reagent were averaged and shown as vertical lines paralleling the index scale. As illustrated in figure 3, dithizone had the highest average factor and was the only collector tested which recovered 80 percent of the lead at a grade of 60 percent.
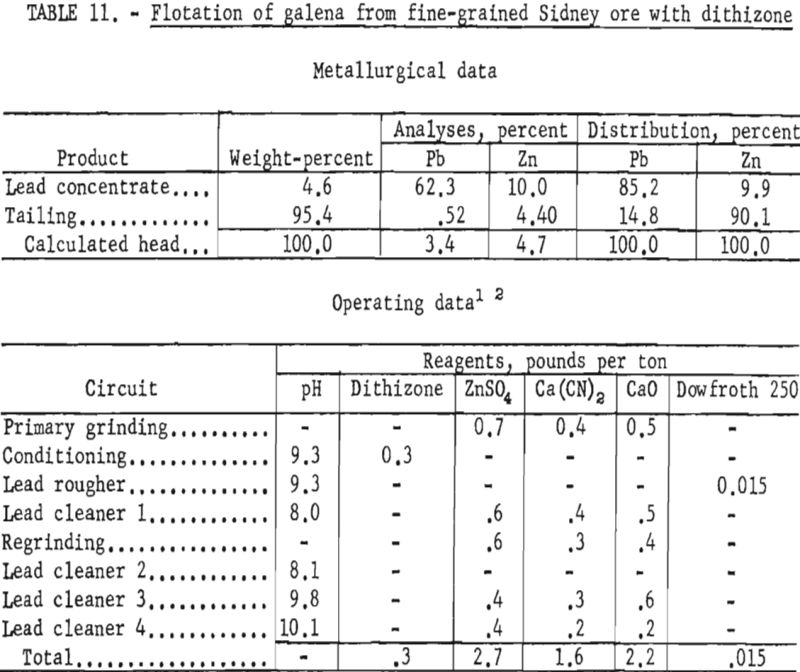
Sidney Ore
Two samples of ore from a deposit being developed by the Sidney Mining Co. were obtained for laboratory studies.
Both samples were mineralogically similar but texturally distinct relative to grain size and association of minerals. The relatively coarse-grained sample contained pyrrhotite and pyrite as well as sericite, quartz, and siderite gangue and had only half as much lead and zinc as reported in the mill feed. Sphalerite was substantially liberated in the 65- to 100-mesh range, whereas galena was locked with gangue in the minus 200-mesh fraction. The second sample, collected at a later date, represented ore from another part of the same deposit. Petrographic examination showed that this material was much finer-grained than the first sample and contained microscopic blebs and arborescent intergrowths of galena in sphalerite ranging from 6 to 64 microns.
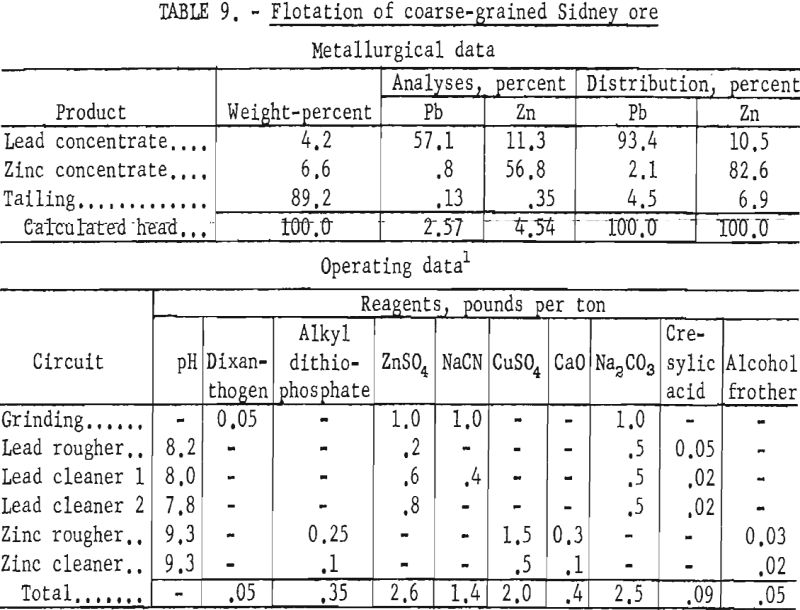
Although the company considered overall recovery of the lead and zinc as satisfactory, it hoped to improve the grade of zinc concentrate. Reported mill results on higher grade ore showed that the zinc concentrate contained about 48 percent Zn and almost 7 percent Pb. Laboratory studies on the coarse sample were directed toward increasing the zinc concentrate grade and improving the lead and zinc recoveries in the respective concentrates.
Laboratory testing showed that grinding the ore to 77 percent minus 325- mesh and floating the sphalerite with an alkyl dithiophosphate at a pulp pH of 9.3 would improve mineral selectivity and recovery. The conditions of the laboratory technique and the results obtained are given in table 9. All cleaner tailings were rejected with the rougher tailing.
Regrinding the lead rougher concentrate to about 18 microns lowered the zinc content of the lead concentrate an average of 1 percent. Lead recovery in the lead concentrate was less, however, as some slime galena was lost to the zinc concentrate.
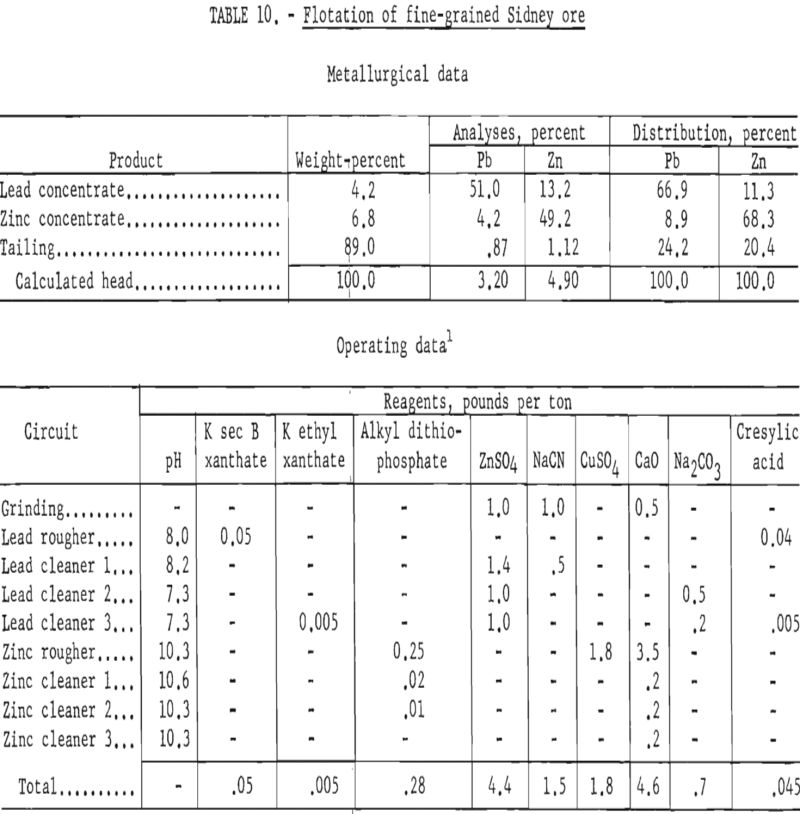
In contrast to the floatability of the first sample, the second, finer-grained ore failed to respond satisfactorily to similar treatment. Table 10 shows the results of a test wherein the same reagent scheme was used but finer grinding was employed.
Further tests on this sample showed that increasing the primary grind to 100 percent minus 400-mesh and regrinding the lead rougher concentrate through 800-mesh increased the lead concentrate grade to about 55 percent Pb at a recovery of about 77 percent. However, the zinc content of the lead concentrate remained about the same.
Examination of test products indicated that, as with the Liberal King ore, contamination of the lead concentrate and loss of lead to the tailings was not due entirely to locking of particles. Although some of the sphalerite particles contained opaque inclusions of approximately 5 microns, galena and sphalerite were essentially liberated from each other and from gangue minerals when the material was reground through 9 microns.
Flotation of the zinc minerals at this finer particle size proved feasible. By applying the reagent scheme shown in table 10 for zinc flotation, the following range of zinc concentrate grades and recoveries were obtained:
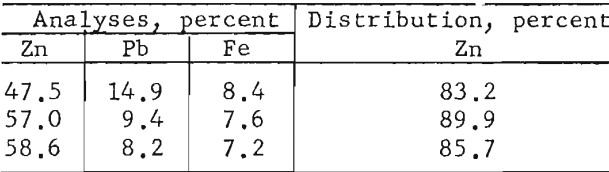
In general, zinc concentrate grades and recoveries varied according to the amount of zinc remaining in the lead concentrate and the number of clean-ing stejjs used in the zinc circuit.
Since flotation of the zinc mineral presented no special problem, studies were directed toward galena concentration and recovery. When conventional reagents failed to provide the desired selectivity at fine particle sizes, tests were made to study the effect of dithizone as a galena collector on this ore sample. Tests on reground lead rougher concentrates at a particle size of 100 percent minus 1,600-mesh, with most of the particles from 3 to 5 microns, gave improved grade and recovery of lead when dithizone was used as a collector. As shown in table 11, the grade and recovery of lead in the lead concentrate were each increased about 7 percent; also, the zinc content of the lead concentrate was reduced about 3 percent as compared with previous tests.
Although the tests indicated that separation of these finely disseminated minerals could be made, the comminution needed for separation is believed to be presently uneconomical.
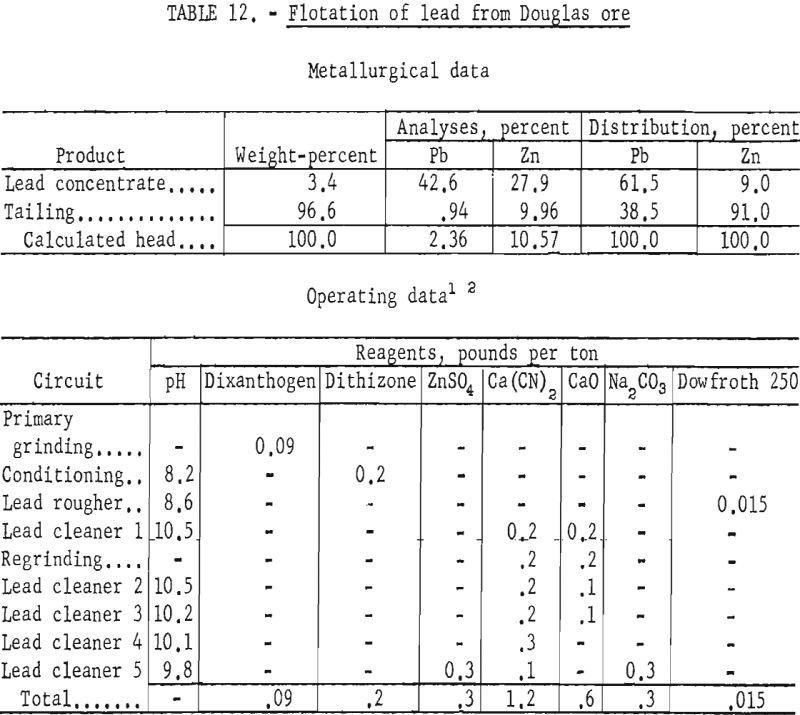
Douglas Ore
The Douglas ore sample represented a refractory portion of ore from a deposit being mined by the Douglas Leasing Co.
Examination and analyses by microscopic and chemical means revealed an even more complex association of the sulfides, primarily sphalerite, galena, and pyrrhotite, than noted in the other samples. Relatively large amounts of zinc and iron minerals were present in the sample in comparison to the amount of galena. The ratio of total zinc and iron to lead was approximately five to one, and the two base-metal minerals were even finer grained than in the other ores. Although a small amount of galena was liberated from sphalerite and the quartz-siderite-sericite gangue in the 150- to 200-mesh sizes, a major portion was locked with sphalerite as inclusions of less than 1 to as much as 20 microns.
The Douglas ore proved to be the most refractory to concentration of any of the samples tested in the laboratory. No lead concentrate made equaled the grade and recovery of the best products from treatment of the other lead-zinc ores. Laboratory studies, employing grinding through 150- or 200-mesh on the fine-grained sample, resulted in lead concentrates of only 26-percent grade at 66-percent recovery. By contrast, zinc concentrates were produced which analyzed 51-percent grade at 78-percent recovery.
The best lead concentrates produced from this ore were obtained by primary grinding of the sample through 400-mesh and regrinding the lead rougher concentrate through 1,600-mesh. Dixanthogen (added in the grinding circuit) and dithizone (introduced during conditioning) were used as galena collectors in flotation. Although dithizone was the most selective galena promoter used, concentrate grades produced still contained only 41 to 43 percent lead, whereas lead recoveries ranged from 66 to 69 percent. In no test was the zinc content of the lead concentrate below 25 percent Zn. Table 12 shows the conditions and results of one of the most satisfactory schemes.
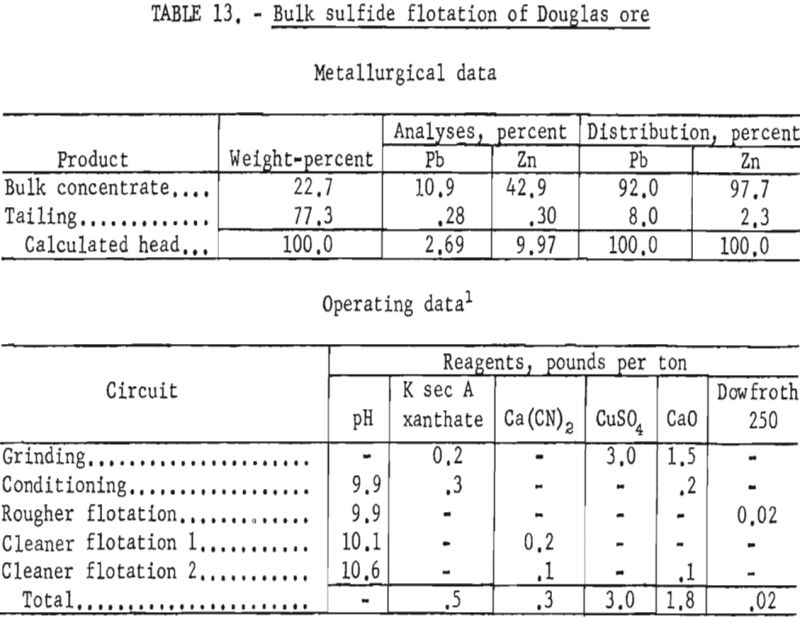
Since the extremely intimate association of sulfide minerals precluded separation of lead and zinc by physical means, bulk-flotation tests were made to determine maximum mineral recovery. These tests resulted in bulk sulfide concentrates containing 90 to 92 percent of the lead and 95 to 98 percent of the zinc. The concentrates contained from 10 to 12 percent Pb and 40 to 43 percent Zn. Table 13 shows the reagent scheme and results of bulk sulfide flotation of the Douglas ore sample. Although such a bulk concentrate would likely be of low value at present smelter schedules for payments and penalties, it would perhaps be suitable as feed for other refinement methods such as selective volatilization.