Tests on electrorefining of yttrium were performed in LiCl-YCl3 electrolytes at 710° C, and the deposits were subjected to vacuum-distillation at 880°C to remove salt from the metal.
A comparison of the analyses of the anode feed and the product metal indicates that all metallic impurities were substantially lowered, but oxygen was reduced appreciably only in metal produced from electrolytes containing 5.4 mol percent YCl3 or less. Variation in initial cathode current density did not noticeably affect the impurity content of the electrorefined metal.
Yttrium possesses properties that are of interest in nuclear applications and in forming alloys for high-temperature uses. Since successful methods had been developed to prepare this metal in quantities, attention has been directed toward purification methods to obtain yttrium in high-purity form for the study of its properties and the evaluation of its commercial application.
Habermann and Daane distilled the metal to obtain a product containing less than 150 ppm oxygen. Williams and Huffine employed solid-state electrolysis to segregate the interstitial impurities. Anable and Beall studied purification by electron-beam melting and reported that yttrium could not be purified by direct heating or by the doping technique they employed.
In our study of purification of yttrium by fused-salt electrorefining, the affinity of yttrium for oxygen was given major consideration, and emphasis was placed on selecting equipment and procedures which would minimize the contact of the product with air and moisture. Inert-gas atmosphere was employed as a protection against contamination from this source, and vacuum distillation was used to remove salt from the deposited metal.
The composition of electrolyte studied was limited to LiCl and YCl3 because most of the other halide salts are not suitable for the following reasons: The fluoride salts generally require higher temperatures to distill than is practical, and YF3 is difficult to prepare without oxide and oxyfluoride contaminations. Alkaline-earth metal chlorides are poor conductors and also require, with the exception of MgCl2, high temperatures to distill. Other alkali metal chlorides are not stable in the presence of yttrium metal because of the relatively high vapor pressures of their metals at the operating temperatures which, under nonequilibrium conditions, would tend to favor reactions resulting in yttrium reduction of alkali metal chlorides to alkali metals. For example, under equilibrium conditions and one atmosphere of pressure at 1,000° K (approximately the electrorefining temperature) and 1,150° K (approximately the vacuum-distillation temperature), the free energies of reaction are 58 kcal and 54 kcal, respectively, for the following reaction:
Y + 3 KCl → YCl3 + 3 K.
Although positive free-energy changes are indicated for this reaction, the fact that the vapor pressure of potassium even at 1,000° K is nearly equal to one atmosphere makes it highly probably that the reaction would proceed when nonequilibrium conditions exist such as in the electrolytic cell where water-cooled components would condense the potassium vapor and in the vacuum-distillation retort where potassium vapor would be continuously removed.
Experimental Work and Results
Apparatus
The apparatus, shown in figures 1 and 2, was designed to permit the electrorefining and vacuum distillation operations and intermediate handling of the product to be performed under an inert gas, thus minimizing atmospheric contamination. The inert gas chamber had a pair of Buta-Sol gloves, three observation windows, and an air lock. To the bottom of the chamber were welded the receiver of the electrorefining cell and the extension of the vacuum-distillation retort with their openings inside the chamber. At the top of the chamber, directly above the cell, there was a port with a removable cover that had a rubber-sealed fitting with an electrical insulator for the passage of the cathode lead. A hydraulic press was installed in one end of the chamber with the ram of the press sealed through a rubber sleeve.
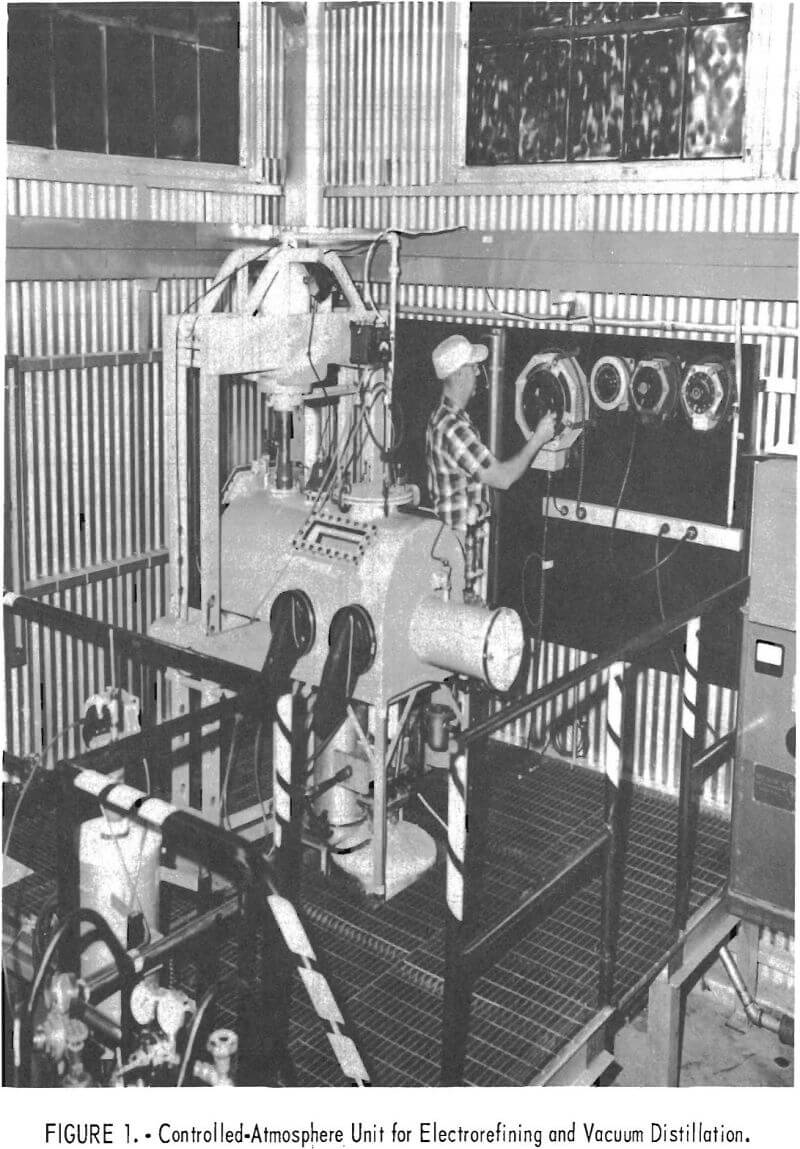
The cell consisted of an electrolyte chamber and a receiver. The electrolyte chamber, 4 inches in diameter and 26 inches long, was made of mild steel. It contained a 16-gage steel liner and was heated in a resistance furnace.
The receiver was connected to the electrolyte chamber through a slide valve. The lid of the receiver sealed the cell body from the inert gas chamber. A fitting with a rubber sleeve and an electrical insulator was provided in the lid to allow the passage of the cathode lead.
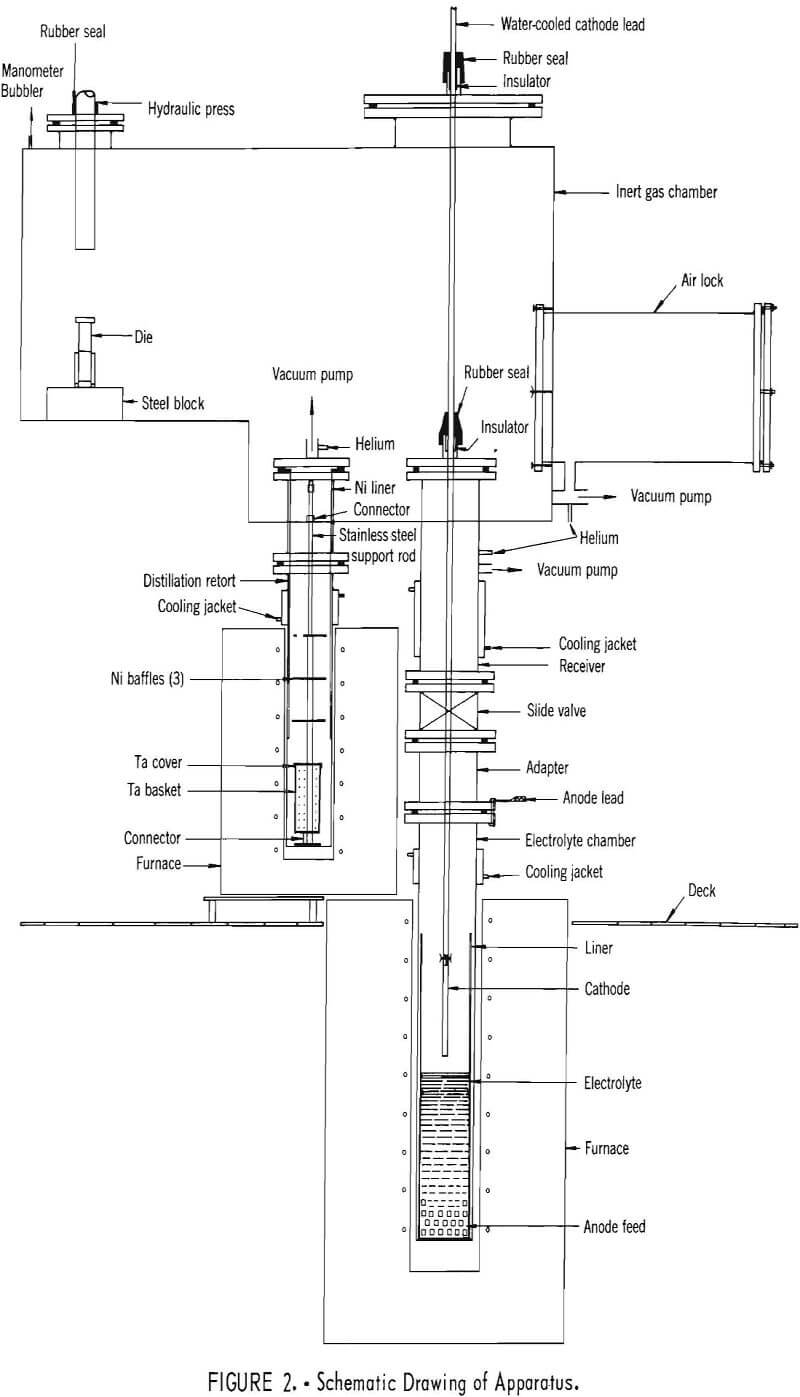
The cathode lead, internally water-cooled, was made of copper tubes. A mild steel rod, ½ inch in diameter, was used as the cathode. The electrolyte chamber with crude yttrium charged in the bottom was connected as the anode. A selenium rectifier supplied the current for electrolysis.
The distillation retort, 3 inches in diameter and 17 inches long, was made of mild steel. It contained a 16-gage nickel liner suspended from the top by a flange. The bottom of the liner extended almost to the top of a tantalum basket which contained the distillation charge.
The basket and cover, fabricated from a perforated tantalum sheet, were threaded through the middle on a stainless steel support rod which was screwed on a nickel bottom plate. Inside the basket the stainless steel rod was covered by a tantalum tube to prevent contamination of the distillation charge. Three nickel baffles were positioned on the rod by spacers above the basket. The whole support rod assembly was set inside the retort which was evacuated from the top through a Dry Ice-acetone cold trap by a two-stage mechanical pump and was heated at the lower end in a resistance furnace.
Procedures
The cell and retort were assembled, degassed, and evacuated to pressures of 20 microns and 10 microns of mercury, respectively. When the vacuum integrity of the systems was considered acceptable, the leak rates for the cell and the retort were approximately 5 micron-liter per minute and 1 micron-liter per minute, respectively.
Approximately 1,200 grams of freshly cut yttrium metal in 2- x 2- x 1- inch pieces were loaded into the electrolyte chamber and degassed, and then 1,600 grams of LiCl were also charged. The yttrium metal was to be used as the anode feed. The chemically pure, anhydrous salt had been previously dried under vacuum, melted, and filtered through a 10-micron sintered stainless steel element.
The cell was sealed and purged of air by evacuating and backfilling with helium three times. In subsequent operations, atmospheric contamination of the cell was prevented by closing the slide valve above the electrolyte chamber whenever the receiver was opened to introduce material to or remove material from the cell, and the receiver was sealed and purged before the slide valve was reopened.
When the cell was heated to 710° C, the YCl3 component of the electrolyte was produced in situ by reacting HCl gas with yttrium metal, 3 HCl + Y → YCl3 + 1-½ H2. The chlorination apparatus, which consisted of a reaction tube and a delivery pipe made of molybdenum, was described in a previous publication. Yttrium metal equal to 1.5 times the stoichiometric requirement was added to the reaction tube, and HCl gas was introduced thereto through the delivery pipe. During the chlorination, the reaction tube was partially immersed in the molten LiCl. The products H2 and YCl3 passed through the perforated bottom of the reaction tube into the melt; the hydrogen was vented out of the cell through an oil bubbler, and the YCl3 was dissolved in the molten LiCl. After 1.3 times the stoichiometric quantity of HCl to yield the desired concentration of YCl3 in the electrolyte was consumed, the chlorination apparatus was removed, and the electrolyte was sampled and analyzed.
After the cathode lead was installed through the fittings in the lid of the cell and the top cover of the inert-gas chamber, the cell and the chamber were sealed and purged of air and electrolysis was performed.
At the end of a deposition cycle, the deposit (fig. 3) was lifted above the electrolyte, allowed to drain, sealed in the receiver, and cooled to room temperature. Approximately 18 deposition cycles, or runs, were made in a batch of electrolyte.
The deposit was stripped from the cathode, loaded into the tantalum basket, and sealed in the distillation retort. The retort was then evacuated and backfilled with purified helium three times before heat and a continuous vacuum were applied.
Distillation of the salt from the metal was accomplished at 880° C for 3 hours, generally with a final pressure of less than 10 microns of mercury. The salt was collected in a ring inside the liner at a level slightly below the external cooling jacket. After distillation, the retort was filled with helium and cooled to room temperature. If preferred, the product metal could be compacted by the press inside the inert-gas chamber before it was sealed in a bottle for removal from the chamber.

Effects of Varying Electrolyte Composition and Initial Cathode Current Density
Electrolytes with the YCl3 concentration varying from 1.7 to 13.4 mol percent were investigated. The solidus temperatures of these salt mixtures ranged from approximately 550° C for the one containing 13.4 mol percent YCl3 to 600° C for the one with 1.7 mol percent YCl3. Preliminary tests indicated that 710° C would be the lowest temperature to allow satisfactory conductance and drainage of salt from deposits for electrolytes with YCl3 concentration in this range; therefore, all the electrorefining tests were made at this temperature.
Initial cathode current densities ranging from 410 to 1,630 amp/sq ft were tested in each concentration of YCl3, and three tests were made at each cathode current density. The order of tests was staggered such that the effects attributable to the age-affected conditions of the electrolyte and anode feed were minimized. The operating data are listed in table 1 and a comparison of the analyses of the anode feed and the electrorefined metal is given in table 2.
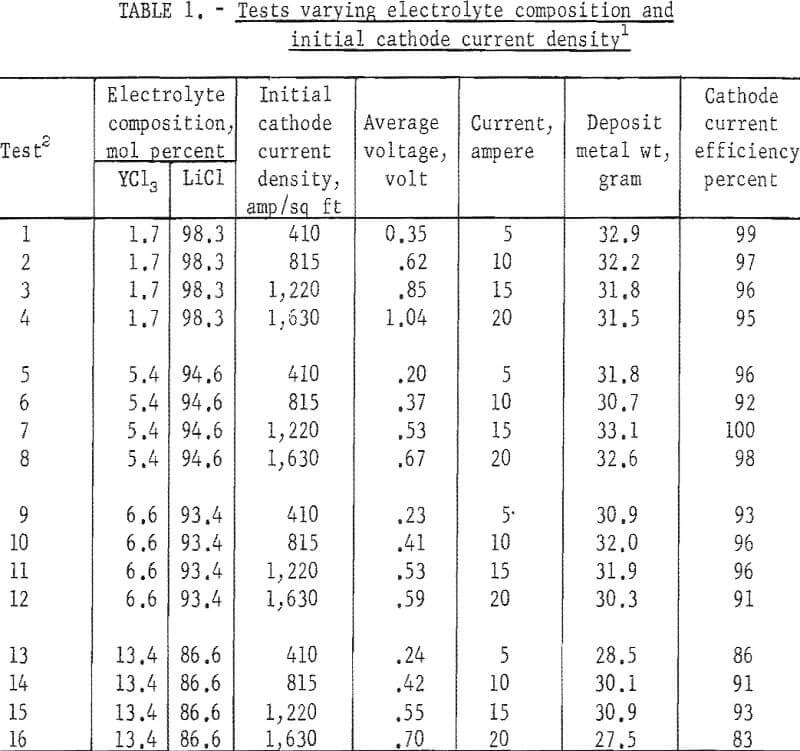
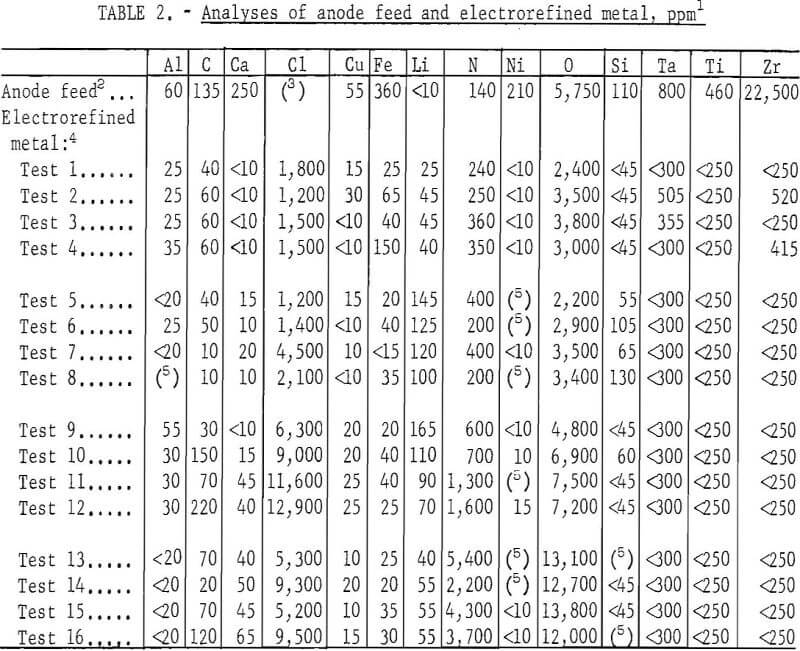
As a whole, the electrolysis was singularly effective in lowering all metallic impurities, but not the interstitial elements. In fact, the nitrogen content of the refined metal was higher than that of the anode feed, and the oxygen content was appreciably lowered only in metal produced from electrolytes containing 5.4 mol percent YCl3 or less.
Variation in initial cathode current density showed no drastic effect on the impurity content of the refined metal. The crystal size of the deposited metal, however, decreased as the initial cathode current density was increased.
Although varying the YCl, concentration of the electrolyte did not appear to affect the metallic impurity content of the refined metal, increasing the YCl3 concentration to above 5.4 mol percent sharply increased the oxygen content of the metal. Nitrogen in the metal generally increased as the YCl3 content of the electrolyte was increased.
The interstitial elements in the refined metal could be partly attributed to the formation of scum on the electrolyte surface during electrolysis. The scum appeared to increase with an increase in YCl3 concentration of the electrolyte. When a deposit was lifted from the electrolyte, it picked up the scum, thus incurring contamination.
When the scum was vacuum-distilled with the deposit, the residual material was brownish and flaky. Analysis of this material indicated that yttrium; chlorine, and oxygen were in major proportions, nitrogen in small quantity, and a trace amount of iron. The presence of yttrium oxychloride (YOCl) was identified by X-ray diffraction, but the state of the nitrogen presence was not clear.
