Table of Contents
The list of pure non-ferrous metals so widely used in modern industry includes many that are produced by electrolytic means, such as copper, zinc, nickel, aluminum, magnesium, lead, sodium, cadmium, calcium and many others. In the case of some metals, ordinary fire or chemical methods of production are important, but in others, 100 per cent of the metal used is produced by electrolysis. The commercial production of electrolytic metals had its origin a century ago when James Elkington, an English electroplater, invented a process for refining copper electrolytically; later, about 1890, aluminum was first produced on a commercial scale by electrolysis, followed by lead in 1905, nickel in 1910, and zinc about 1915.
There are a number of reasons why metals produced by electrolysis have played such an important part as they have in modern industry. In the first place, metals produced in this way are usually exceptionally pure; the purities shown in the following table are obtainable in commercial practice.
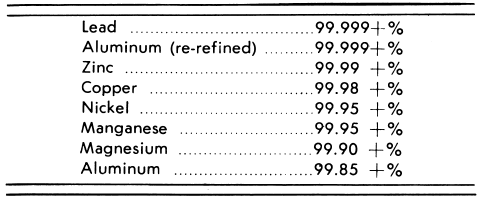
The peculiar properties of high-purity metals include: extraordinary high resistance to corrosion, high malleability, high electrical conductivity, and others of a similar nature. High-purity lead gives exceptional service in chemical plant construction, the zinc die-casting industry depends upon high-grade zinc metal, high conductivity copper is essential in the electrical industry, pure nickel finds a multitude of uses due to its special physical properties, re-refined aluminum is extremely soft and malleable, has a brilliant mirror surface which will not dull or corrode, and is very difficulty soluble in acids or alkalies. Such properties are most readily and economically developed by producing the metals electrolytically.
A second reason for the employment of electrolytic methods is that, often, they provide the most economical method of separating a valuable metal from the gangue, slag, or other metals with which it is combined. Also, in the case of metals standing high in the electromotive series, such as sodium and aluminum, electrolysis provides the only practical means for breaking down the oxidized compounds and preparing the metal in reduced, metallic form.
Basis of Electrolytic Refining Methods
All electrolytic operations depend upon two basic factors: the first is the volume or quantity factor which is related to amperes; the second is the energy or pressure factor which is related to volts.
The quantity of metal deposited from an electrolyte is in accordance with the laws enunciated by Faraday, which state in effect: (1) that the quantity is proportional to the number of ampere-hours, and (2) that a given number of ampere-hours will deposit an equivalent amount of any metal, the equivalent being determined by dividing the atomic weight by the valence. Experiment and calculation prove the validity of Faraday’s laws and show that, if a current of 1000 amperes passes through a cell for one hour (or one ampere for 1000 hours—however one wishes to figure it), the following weights of metal will be deposited if the current—and cell efficiency—is 100 per cent:
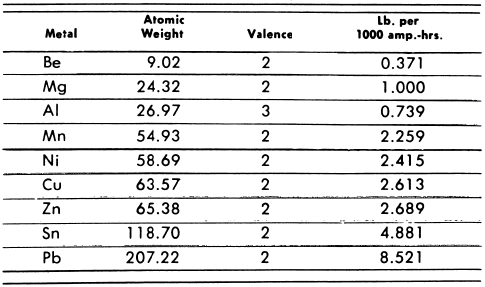
From the table it is evident that the same amount of current that will deposit 8.5 pounds of lead will deposit only 6 ounces of beryllium or 12 ounces of aluminum. The current efficiency of an electrolytic cell can be calculated by comparing the amount of metal actually deposited by a current passing through the cell for a given time with the amount which should be deposited, according to Faraday’s law, by the same current passing for the same length of time.
The voltage required to operate a cell is a different matter entirely, as it is dependent on several factors such as electrolyte resistance, and contact resistances, as well as the decomposition voltage. The latter factor cannot be simply expressed, but may be said to represent, in electrical units, the heat units which must be supplied to decompose a compound such as copper sulphate or aluminum oxide in such a way that the energy content of the component elements is restored to the level existing before the compound was originally formed. For each compound and electrolyte, a definite potential or pressure must be provided before any current (amperes) will flow—this is the decomposition voltage—and, once it is reached, the current continues to flow at the constant pressure of the decomposition voltage, the metal being deposited according to Faraday’s law. The voltages usually required to cause current to flow through a cell are low in aqueous electrolytes and only slightly higher in molten electrolytes, despite the fact that the resistance of molten electrolytes is sufficient to generate the heat required to keep the electrolyte fluid. The conditions of electrolysis vary so greatly for different metals and different electrolytes that no set relations can be given, though it may be noted that cell voltages vary from about 0.25 v. for electrolytic copper refining with soluble anodes to about 5 v. for aluminum reduction with carbon electrodes.
The power requirement (W) for electrolytic production of metals is determined, basically, by the ampere- hour (I) requirement, combined with the cell-voltage (E). From the relation
W = EI
Watts = Volts x Amperes
it is obvious that the two factors are multiplied. It is possible to calculate I accurately, but as E is dependent on a number of factors, W can be determined only by trial. As metal is deposited by direct current, the power requirement of an electrolytic plant must also include line-losses, rectifier losses and transformer losses. The average power or energy requirements for some metals is as follows:
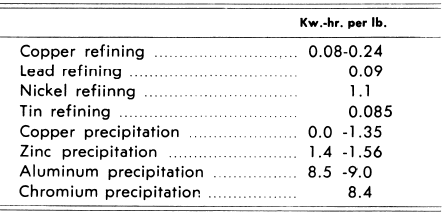 The above figures represent operating data and include cell-resistance, ampere efficiency, etc.
The above figures represent operating data and include cell-resistance, ampere efficiency, etc.
Electro-refining and Electro-winning
The field of electrolytic production of metals can be conveniently separated in two main divisions which may be termed, briefly, electro-refining and electro-winning. The operations are similar in that both employ direct current, which passes from anodes to cathodes in a suitably arranged cell containing an electrolyte, and in that in both types of operation, pure metal is deposited on the cathode. The essential difference between the two processes is that in electro-refining an impure alloy anode is used which is dissolved by the action of the current, thus replenishing in the electrolyte an amount of metal approximately equivalent to the amount deposited at the cathode: in electro-winning, however, the anode is made of some material such as lead or carbon which is insoluble in the electrolyte, making it necessary to replenish the metal content of the electrolyte by the addition of a metal compound which is soluble in the electrolyte. Though the amount of refined metal deposited per ampere-hour is the same in both processes, electro-winning requires more energy per pound of metal deposited than does electro-refining, and cell voltages are therefore much higher for electro-winning operations, with resulting higher power consumption (note second half of table above). In electro-refining, when the cell is operating properly, no gases are evolved at either anode or cathode: in electro-winning, a gas is always evolved at the anode when the cell is operating. (Commonly, oxygen or chlorine is thus produced.)
Electro-refining
Usually the object of electrolytic refining is to separate one metal in pure form from an alloy containing a high percentage of the desired metal, copper for instance, and a number of other metals dissolved in the copper or intimately mixed with it. The impurities can be classified in two groups: (a) those metals which can be more easily oxidized than copper, e.g., iron, nickel, and (b) those metals less easily oxidized than copper —e.g., gold, silver.
Copper:
In the actual copper-refining operation, the impure alloy of copper, nickel, gold, etc., is cast in a thin, flat plate constituting the anode: the cathode is usually a sheet of pure electrolytic copper. Anodes and cathodes (30 to 40 of each) are immersed in a cell containing copper sulphate and sulphuric acid which serves as an electrolyte. The action of the electric current can be easily visualized of it is recalled that the action at the anode is always oxidizing and at the cathode reducing. The passage of the current tends to oxidize iron, nickel, and copper at the anode. No gold or silver will oxidize at the anode as long as there is metallic copper present. The metal oxides are soluble in the sulphuric acid of the electrolyte — and, as sulphates, are free to move to the cathode. At the cathode the copper sulphate, being more easily reduced than other sulphates present, is broken down to copper metal and sulphuric acid. The sulphuric acid returns to the anode to dissolve more copper, thus completing the cycle. None of the other sulphates in the electrolyte will be decomposed at the cathode as long as there is copper sulphate for the current to work on. In this way, the copper is separated by preferential solution from gold and silver which remain at the anode in metallic form so finely divided that they form a slime; it is separated from iron and nickel at the cathode by preferential decomposition of the sulphate.
The energy relationships in a cell of this type are characteristic of those in all cells where soluble anodes are used. When a compound such as copper sulphate is formed from copper, oxygen, and SO3, a certain amount of energy is released or made available in the form of heat or equivalent electrical units. Conversely, when copper sulphate is decomposed to form copper, oxygen, and SO3 exactly the reverse is true—an equal amount of energy (heat or electrical) is absorbed. In the cell with soluble anodes, both reactions are proceeding at the same time, so that energy required for decomposition is derived from the energy resulting from combination, the final result being that no energy is absorbed or dissipated. Hence, the only new electrical energy required to operate the soluble anode cell is represented by the voltage necessary to overcome polarization, cell resistance, etc. This voltage is relatively small in the case of copper sulphate—sulphuric acid electrolytes.
Other impure metals are refined in exactly the same way, though it is necessary to adjust the electrolytes and other operating conditions to suit each individual metal, the essential requirement being that the electrolyte must be composed of solution of a soluble salt of the metal to be refined.
Lead:
A number of electrolytic lead plants are in operation, separating lead from copper, antimony, bismuth, gold, silver, etc. In this operation the anode is only 98 per cent lead, with antimony, copper, gold, silver, etc., present as impurities. A solution of lead fluosilicate and hydro-fluosilicic acid forms the electrolyte—sulphuric acid would not do, as lead sulphate is insoluble. The action in the cell is, with obvious modifications, identical with that in the copper refining cell, with the exception that any tin present in the anode is dissolved with the lead and deposited on the cathode.
Nickel:
Nickel is refined electrolytically from metallic nickel or nickel sulphide anodes containing as impurities iron, copper, gold, silver, platinum, palladium etc. The electrolyte contains, principally, nickel sulphate in a nearly neutral solution. Extraordinary precautions must be taken to ensure that the electrolyte at the cathode is free of copper, iron, and other impurities. Each cathode is suspended in an individual porous- walled compartment to which pure electrolyte is fed at a predetermined rate. The impure electrolyte leaving the anodes is removed from the cell and is purified by removal of iron, copper, etc., before being returned to the cathode compartments for precipitation of pure nickel on the cathode. Otherwise, the basis of operations resembles that in copper refining.
Silver and Gold:
Silver and gold are commonly refined electrolytically—a neutral silver nitrate solution is the electrolyte for silver refining, and gold chloride solution for gold refining.
Precious Metals
The anodes used in refining base metals — nickel, copper, lead—by electrolysis usually contain gold, silver, platinum, palladium, etc., as well as selenium and tellurium in some amount. In every case, these metals, being less easily oxidized than the principal metal constituting the anode, are not dissolved by the electrolytic action, and remain at the anode in finely divided form. As the principal metal—copper, for instance—is largely dissolved, the weight of anode slime is small in relation to the weight of the original anode, and the previous metals (and selenium and tellurium) are concentrated in the slime, along with antimony, arsenic, lead, bismuth, etc.
The methods employed for separating and recovering the metals present in the slimes are determined by the composition of the slime in each individual case. The slime may be roasted, treated chemically, melted and re-electrolyzed, oxidized, leached, etc., with the ultimate object of recovering antimony, bismuth, selenium, tellurium and arsenic as by-products and producing an alloy composed principally of precious metals— usually gold and silver, though platinum metals are often present in important amounts. The precious-metal alloys are then treated chemically and by electrolytic refining methods to separate and recover the individual metals.
The recovery of precious metals and by-product metals is an important phase of the electrolytic refining of the common base metals.
Electro-winning
The object of electro-winning operations is to decompose an oxidized compound of a metal and deposit a pure metal on the cathode, not only separating the metal from gangue, but actually reducing it to metallic form for the first time. (Normally, in electro-refining, the metal appears in metallic form in the anode, the necessary reduction having been affected by smelting or some similar operation.) Electro-winning thus provides a method of producing metal from an ore without smelting.
The operation of an electro-winning process requires that the metal to be recovered be in some form in which it can be dissolved in a suitable electrolyte, while the anodes must be composed of some material insoluble in the electrolyte. The cathodes may have any suitable form, but consist usually of the metal it is sought to recover.
Zinc:
The action in the cell is relatively easily explained, and can perhaps best be illustrated by the production of electrolytic zinc. In this operation, a neutral solution of zinc sulphate is fed to the cell which contains a series of lead anodes and zinc-covered cathodes. The electric current leaving the anode would tend to oxidize the lead, but as lead oxide is not soluble in sulphuric acid, the surface of the anode becomes inert and water from the electrolyte is decomposed to liberate oxygen gas and hydrogen ions. At the cathode, the reducing action of the current decomposes zinc sulphate, depositing metallic zinc on the cathode and leaving sulphate ions in solution. Thus neutral zinc sulphate solution entering the cell is converted to metallic zinc, oxygen and sulphuric acid (hydrogen ions and sulphate ions). The sulphuric acid so generated is overflowed from the cell and is used to dissolve zinc oxide made by roasting zinc sulphide concentrates.
The acid dissolves zinc oxide in preference to iron oxide, but some other oxides also dissolve in the acid, and the solution must be purified before being returned to the cell as neutral zinc sulphate, where the cycle is repeated.
The essence of the operation is that zinc oxide is chemically dissolved, enters the cell where oxygen and zinc are produced and acid regenerated to dissolve more oxide. Basically, nothing more or less has happened than that the cell provides a mechanism for the electric current to supply the energy necessary to decompose ZnO to its elements—Zn and O. This is the fundamental difference between electro-winning and electro-refining, and it is this energy requirement that necessitates higher voltages in the electro-winning operation. As electricity is purchased on the basis of volts and amperes, and as the ampere requirement is constant (Faraday’s Law), the higher voltage means higher power cost. For this reason, electro-winning operations are almost invariably located in low-cost power areas.
Copper, Nickel, Cadmium:
Some of the world’s copper is produced by leaching and electrolysis. The basis is exactly the same as described for zinc—even to the use of a sulphate—sulphuric acid electrolyte. Nickel is not produced by this means, due to the insolubility of nickel oxide. However, the process could easily be employed if nickel carbonate, for example, were available as a raw material. Cadmium is recovered from zinc plant by-products by an electro-winning process resembling the zinc process.
Aluminum, Magnesium: Aluminum and magnesium are both produced by electro-winning methods—100 per cent of the aluminum and most of the magnesium is made in this way. Neither of these metals can be reduced from compounds in an aqueous solution, so molten salt electrolytes are employed. Basically, the operations are the same as those already described, but the techniques are quite different.
In the case of aluminum, the anode is composed of carbon, the cathode is a bath of molten aluminum at the bottom of the cell, and the electrolyte consists of molten sodium-aluminum fluorides in which is dissolved pure Al2O3. The pure Al2O3 is prepared by chemical means and is added to the cell at regular intervals. The electric current decomposes the Al2O3, depositing pure aluminum on the cathode, while the oxygen released at the anode burns the carbon to form CO2 which escapes from the cell. The anode is thus insoluble in the electrolyte, but is constantly being consumed and must be continuously renewed. There are no by-products from the cell.
The production of magnesium is rather different. The most satisfactory electrolyte is molten magnesium chloride mixed with an alkayli-chloride, usually sodium chloride. A graphite anode is used and a steel cathode —the latter serving merely to conduct the current away from the cell. Magnesium metal is released from the chloride at the surface of the steel cathode and, as the metal has a lower specific gravity than the chloride, it rises to the surface of the molten electrolyte, there to be removed by ladling. The chlorine generated at the anode is conducted through pipelines to a furnace where it is reacted with carbon and MgO to produce anhydrous MgCl2, which in turn is charged to an electrolytic cell, thus completing the cycle. Where the Mg is produced from seawater, the chlorine is reduced to hydrochloric acid which is used for conversion of Mg(OH)2 to MgCl2.
Precious Metals
In electro-winning processes, only those metals soluble in the leach solution are recovered by electrolysis. If an ore contains precious metals, it is most unlikely that they will be recovered, as, for instance, the dilute sulphuric acid used for leaching copper and zinc ores and calcines do not dissolve gold or silver. The application of electro-winning methods is, therefore, usually limited to ores which do not contain precious metals in sufficient amount to justify their recovery, to light-metal ores which usually contain no precious metals, or to relatively high-grade materials such as concentrate, mattes, and by-products in which the precious metal content is so high that the residue from leaching can be further processed by smelting methods to recover the gold and silver present.
Source: This article is a reproduction of an excerpt of “In the Public Domain” documents held in 911Metallurgy Corp’s private library.
