Table of Contents
- Effect of Silica
- Effect of Calcium Compounds
- Effect of Magnesium Compounds
- Effect of Iron
- Effect of Nickel and Cobalt
- Effect of Aluminium
- Effect of Zinc
- Effect of Arsenic
- Effect of Copper
- Effect of Lead
- Effect of Mercury
- Effect of Bismuth and Tin
- Effect of Selenium
- Effect of Tellurium
- Effect of Silver
- Washing Out the Soluble Salts
- Treatment of the Insoluble Residue
- Amalgamation of Gold Slimes during the Refining Process
- Experimental Work on Various Gold Slimes
The richer the slimes are in gold the easier it is to treat them. In the majority of cyanide plants not enough care is taken to eliminate mechanical impurities before the solutions pass into the zinc boxes. No silica or insoluble substance should be allowed to pass into the precipitating box. Further, some salts are precipitated from a dilute solution which dissolve in a strong solution of potassium cyanide. If a strong solution of potassium cyanide is run through the box before cleaning up, many extraneous salts are dissolved and removed, and the precipitate rendered much cleaner.
Effect of Silica
When siliceous material in a fine state of division is present the first effect is to dilute the fused mass, and prevent the gold from running together; the second is to cause the adhesion of very finely divided gold to each particle of siliceous matter, rendering it a pink colour throughout. In fact such classes of insoluble materials appear to act in a similar manner towards finely divided gold as certain precipitates do towards coloured substances which form pigments or lakes. The formation of the purple of Cassius with tin oxide probably is due to the same cause. The finely divided silica, with its gold attached, interferes mechanically with the subsidence of the gold. It is, therefore, highly desirable to totally exclude it from the zinc boxes.
Effect of Calcium Compounds
When lime is present in an ore either through being added to neutralise acids, or to flocculate slimes in decantation processes, or when the ore contains calcite and becomes roasted, some salts of calcium, as well as calcium hydroxide, are present in solution. If a solution becomes saturated with calcium sulphate the latter is apt to be deposited on any surface it comes in contact with. The launders down which the solutions run will often show long crystals of gypsum standing out at right angles to the course of flow of the liquid. In the zinc boxes also precipitation is apt to take place, although to a lesser degree. Should the working solution be highly saline the gypsum is not precipitated so readily since it is dissolved much more freely in such solutions. Calcium sulphate is not a common impurity, but it occurs in the Kalgoorlie mines, and forms, when the ores are roasted, if calcium carbonate is present; this latter salt is usually precipitated in the box itself, due to the interaction of the lime solution, and the carbon dioxide evolved from the decomposition of the cyanide.
When calcium carbonate or calcium hydroxide is present, it is easily removed with dilute hydrochloric acid. The acid will attack and remove either of these substances before it attacks the zinc. If a large amount of this material is present it is better removed by first washing it with dilute hydrochloric acid, and then decanting the clear solution and washing the calcium chloride out of the slimes.
If the calcium compounds are not removed they become transformed to sulphate in the subsequent operation, and as the material is so slightly soluble in water the bulk of it remains. It has the same mechanical effect as silica in causing some of the gold to remain in a finely divided state, and in retarding filtration; it also has a bad effect in rendering the fused cake more difficult to dissolve in water, since a compound analogous to glauberite forms. (Na2 SO4. CaS04).
Calcium sulphate might be dissolved out of the solution with sodium thiosulphate, or by heating it with sulphur and water, thereby forming calcium thiosulphate, which is more readily soluble. The trouble with regard to this mode of procedure was that some gold also dissolved by the action of the thiosulphate. It is much simpler to eliminate the calcium compounds by a preliminary treatment. The nitre cake used should be as free as possible from this substance.
Effect of Magnesium Compounds
The preliminary treatment with sulphuric acid removes the magnesium as sulphate, consequently it does not interfere, although it is frequently found encrusting the zinc filaments in the form of hydroxide.
Effect of Iron
Iron as such is not precipitated on zinc shavings in an alkaline solution, but ferro-cyanides form, and are precipitated, or crystallise in the box, and the iron present is due to them. The preliminary treatment with strong sulphuric acid transforms these into sulphates and carbon monoxide, according to the following equation:—
K4Fe (CN)6 + 8H2SO4 + 6H2O = 4KHS04+ FeSO4 3 (NH4)2 SO4 + 6CO.
The ferrous sulphate becomes oxidised at a higher temperature to form ferric sulphate.
2Fe SO4 + H2 SO4 + O =Fe2 (SO4)3 + H2O.
The oxygen necessary may come from the air or from the sulphuric acid itself. On further heating basic sulphate of iron forms, or if the temperature is very high ferric oxide is formed and sulphur trioxide evolved.
Fe2 (SO4)3 = Fe2 O3+ 3SO3.
Usually ferric sulphate is left. When iron vessels are used a considerable quantity is mixed with the fused cake. If the pyrosulphate is totally decomposed then there is a great tendency for the ferric sulphate to be precipitated as basic ferric sulphate, when hot water is added for washing. This is objectionable, since it forms a slimy mass, which retards washing, filtration, and also the solution of the cake. It is therefore better, when much iron is present, to keep it in solution by adding either a small amount of sulphuric acid or nitre cake near the close.
Effect of Nickel and Cobalt
These metals pass into solution readily as sulphates.
Effect of Aluminium
Small amounts of alumina are dissolved from the sediment, which enters the zinc box. This behaves,in a similar manner to ferric sulphate.
Effect of Zinc
This metal is one of the chief impurities in the original slime. The most economical way of removing it is by means of dilute acid. As before stated, the strong acid oxidises, as well as dissolves, the metal.
Zn + 2H2SO4 = ZnSO4 + SO2 + 2H2O.
Further, zinc at a high temperature, will oxidise at the expense of sulphates present, reducing the latter to sulphides. It is not desirable to have the metal present, especially if it is at all coarse. If the slimes are finely divided, the zinc and zinc salts present are transformed to sulphates. It is not objectionable in this state, since it will stand a high temperature without decomposition, and in this way protects silver sulphate from splitting up.
Effect of Arsenic
Arsenic often passes into solution when ores containing this element are dealt with. It is precipitated from alkaline, solutions on the zinc. The element itself, when treated with dilute sulphuric acid, is not affected, but in solution, in the presence of zinc, or alloyed with zinc, arseniuretted hydrogen is evolved. This is one of the most poisonous gases to be guarded against when cleaning up the zinc gold slimes.
When treated with strong sulphuric acid it is oxidised, and passes into solution. If the solution is neutral, or nearly so, it will form insoluble basic salts, with ferric or aluminium sulphate. Arsenic may be expelled as a volatile chloride by adding sufficient salt, when the mixture is heated with strong sulphuric acid.
Effect of Antimony
Antimony behaves similarly to arsenic with dilute sulphuric acid. With strong sulphuric acid it passes into solution, but is almost wholly precipitated when water is added to the solution. It can be kept in solution by means of tartaric acid, but it would probably be easier to remove it by a preliminary fusion with sulphur and carbonate of sodium when the whole of the antimony, as well as the arsenic, would be rendered soluble.
Effect of Copper
When copper is exceedingly finely divided no modification of the method is necessary. It may be sometimes desirable to dissolve out copper if present in excessive quantity by means of sulphuric acid and air, when only half the amount of sulphuric acid will be required. For example:—
Cu + 2H2SO4=Cu SO4+2H2O + SO2.
CU + H2 S04 + O = CU SO4+H2O.
Bismuth and part of the arsenic are removed by this method of treatment. If a certain amount of sulphate of copper is formed in the nitre cake process, the solution of the silver is facilitated, for sulphate of silver is more readily soluble in solutions of copper sulphate than in water. Like other sulphates before described it decomposes before silver sulphate, so that the presence of copper sulphate in the final melt is a guarantee that the silver sulphate has not decomposed.
Effect of Lead
Lead is objectionable in that it forms a sulphate insoluble in water. When fused in pyrosulphate it appears to pass into solution. It does not prevent the segregation of the gold. When, after washing salts out of the fused mass, the residual gold is smelted, lead itself tends to become reduced, and pass into the amalgam or smelted gold. Sulphate of lead, as well as sulphate of lime, can be removed by first treating the residues containing these salts with carbonate of sodium and stirring well until the solution becomes alkaline.
Na2C03 + PbSO4= Na2SO4 + PbCO3
The sulphate of sodium, as well as any carbonate added in excess, is then removed by decanting the clear solution; after decantation the soluble sulphate should be washed out by filtration. When the washings come away neutral the action may be considered complete. If dilute nitric acid is now added, until the liquid remains acid, the carbonates will have been converted into nitrates.
PbC03 + 2HNO3 = Pb(NO3) + H2O + CO2
The lead nitrate can be washed out, and the solution, after neutralising any excess of nitric acid with sodium carbonate, can be used instead of lead acetate, either as a corrective to soluble sulphide in an ore, or to aid in precipitating gold in the zinc boxes. The greater part of the calcium is also removed by this operation, and the gold, previously present as a pink powder, becomes darker, and is very finely divided.
Effect of Mercury
When slimes containing mercury are treated by this method mercuric sulphate forms, but is split up into mercury and sulphur trioxide, both of which escape.
Effect of Bismuth and Tin
No slimes from the zinc boxes were found to contain these elements. The former occurs in the slimes produced from the electrolysis of copper. It becomes sulphate like other metals, but tends to separate out as a basic salt when being washed with water. It may be retained in solution by increasing the amount of acid present, or it may be allowed to settle with the gold, and recovered from the same by the addition of more acid.
Effect of Selenium
Only one sample of zinc box slimes containing an appreciable quantity of selenium was dealt with. It came from the Waihi Mine, N.Z. When the slimes are smelted direct with fluxes, about one or two per cent, of selenium passes into the bullion. This has proved very difficult to remove. It was found in the preliminary drying and heating with sulphuric acid and nitre cake that the selenium escaped as a brown vapour, rapidly condensing to a red amorphous powder, and that it was wholly expelled by heating the slimes with excess of nitre cake.
Effect of Tellurium
This element, although so abundant in the auriferous ore at Kalgoorlie, does not appear to enter into the composition of the ordinary cyanide gold slimes. It is scarcely credible that the tellurium is oxidised and wholly volatilised by roasting. Slimes from the bromo-cyanide process contain a considerable amount. On being heated with strong sulphuric acid, followed by treatment with nitre cake, tellurium is oxidised to tellurous acid. This tends to separate out when washed with water, but is readily soluble in acids. It can therefore be removed after the same manner as bismuth, or by applying an alkaline wash.
Effect of Silver
This metal has already been dealt with. There is no trouble about converting the whole of the metal present, no matter whether present in large or small quantity, into sulphate; but there is much trouble in dissolving large amounts of silver sulphate in water. One simple way of so doing is to place the fused mass on a filter spread over a wooden grid and suspended just below the surface of the liquor in a wooden vat. If the solution in the vat is kept boiling by means of steam the silver sulphate will pass into solution. On allowing any fine gold to subside, the solution may be decanted off and the silver precipitated on copper or iron, in the usual manner.
In order to find out the solubility of silver sulphate when produced in treatment of slimes, several tests were made. The weight of slimes taken was 1508 grains; these were taken direct from the zinc boxes. They contained gold and silver in the proportion of 4 of silver to 1 of gold. This parcel was moistened with 50 c.c. of chamber sulphuric acid, 60 per cent, strength, and 50 c.c. of water, and 200 grains of nitre cake. The mixture was heated to dull redness. While still hot 2000 grains of nitre cake were added, and the mass heated until the whole was in fusion. The weight of the fused cake was 3100 grains; this was broken into coarse pieces and placed in cold water, and steam was blown in until the whole volume was 500 c.c. This was allowed to stand for a short time, and the clear liquor decanted, and the operation was repeated several times.
10 c.c. of the first decanted liquor gave 2.160 grains silver.
10 c.c. of the second decanted liquor gave 1.750 grains silver.
10 c.c. of the third decanted liquor gave 1.050 grains silver.
10 c.c of the fourth decanted liquor gave 0.772 grains silver.
The silver was wholly in solution after the last addition of water. The weight of silver recovered was 286 grains. The weight of gold recovered was 73.1 grains, assaying 994. The insoluble residue from this example weighed 320.6 grains. Another test was made on slimes containing 11.53 per cent, gold and 3.45 per cent, silver. The charge taken was:
Slimes………………………………………………………………………….1000 grains.
Sulphuric acid………………………………………………………………..200 grains.
Nitre cake……………………………………………………………………..100 grains.
Water to moisten.
The product was gradually heated to dull redness, when 2000 grains of nitre cake were added, and the mass heated to full redness. After cooling the fused mass was inverted into a large porcelain dish containing 100 c.c. of water. After some hours the fused cake dropped out of the vessel in which the fusion was made into the lower one; large crystals of sodium sulphate had formed. After decanting the clear liquid 140 c.c. of water were added, and after stirring and allowing to settle, this was again decanted; the operation was repeated for the third time with 100 c.c. of water. Samples containing 10 c.c. were withdrawn from each solution, decanted and tested, the results being as follow:—
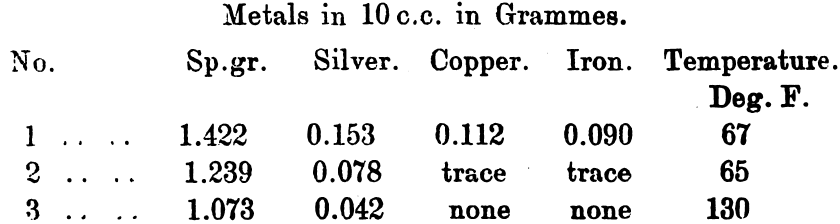
After the third addition the whole of the silver was in solution. In actual practice the amount of silver in solution varies with the amount of acid present, the sulphates and the temperature; as a rule a hot solution contains a minimum of 1.35 per cent., or 13.5oz. per cubic foot. All hot saturated solutions will deposit about half of their silver as hard yellow crystals, of normal sulphate of silver on cooling.
Effect of adding strong sulphuric acid to dissolve silver sulphate : When sulphuric acid is added to the fused cake, and the whole heated, it will melt at a low temperature; but the salt is too dense to allow of a perfect settlement of gold in most cases. The sulphuric acid reforms the sodium bisulphate, and if this is washed out without any attempt to recover it the acid would be lost. In most cases, even when silver has been removed from it, it is too impure to be used when diluted for dissolving zinc from the slimes, since the metals dissolved, such as copper, would at once be precipitated.
If sulphuric is added after the sodium sulphate and most of the base metals have been washed out, the silver remaining dissolves readily enough, but the material remaining usually contains so much impurity in the form of silica and insoluble sulphates that the supernatant liquid becomes quite milky, and cannot readily be decanted from the gold. If the slimes were free from admixed foreign substances there would be as little difficulty as there is in parting by the well- known sulphuric acid process. The use of ammonia as a solvent for silver sulphate, or silver chloride which may have formed is preferable to sulphuric acid, but all acid and soluble salts must be removed before it is applied, since it will yield voluminous and very often slimy precipitates. Since both the ammonia and the silver may be readily recovered from such solutions, it can be used where ammonia is available. It is also advisable to use a small amount of it in ordinary cases, even when the soluble sulphates of silver have been washed out in order to readily remove any chloride or salt or silver which dissolves with difficulty in water, but which dissolves readily in ammonia.
Washing Out the Soluble Salts
Since wood, after being used for some time seems to have no action in reducing silver sulphate from its solutions, vessels constructed of it may be used for the solution of the cake. The capacity of the vessel should be in proportion to the amount of silver to be kept in solution. Two systems of solution can be adopted—the former consists in dissolving the whole of the soluble salts in one operation, using just so much water as will make a solution nearly saturated with silver sulphate. By adopting this process, solution is retarded more and more towards the finish, the salts dissolve readily at first, but as solution proceeds they dissolve more and more slowly. The other system is to wash the cake in a series of vessels, each containing a portion of the fused cake. The water would be run over the first, and overflow into the second and so on until it reached the final one. By washing in this way, solution takes place more readily, and the effluent liquor remains saturated with silver sulphate until nearly the close of the operation. As previously described, washing according to the first method is better done in a vat, a grid framework with boarded sides, the latter being dove¬tailed, and the grids fastened with wooden pegs, thin covered with a coarse filter. The framework is hung so as to dip into a vat of water. The fused cake is put in, and steam is blown into the solution through a glass pipe fastened on to a steam pipe by means of a rubber or other flexible joint. The temperature will rise to the boiling point of the solution, which is above that of water. Boiling is thus effected without danger of bumping. The cake dissolves, and the soluble salts and fine material pass through the filter. The solution should be complete, for if small lumps remain they are certain to contain silver sulphate. When solution is complete the steam can be turned off, the fine sediment will rapidly subside, and the clear liquid containing the silver sulphate can be run off into another vessel; if it passes through a filter before reaching the second vat there will be no danger of loss of fine gold. The silver can be recovered by various means from this solution.
When time allows it the solution may be effected in the same manner with cold water. The sodium sulphate dissolves very readily, also the ferric and aluminium sulphates, which are apt to become decomposed by hot water. By leaving the tray in water over night the salts will pass into solution, and diffuse through the filter cloth into the vat. If the cake were placed on the bottom of the vat the liquid around it would remain saturated and solution would go on very slowly, but by having it suspended near the surface of the liquor solution is assisted by gravity.
The other method which suggested itself was based on the principle used when precipitating gold from cyanide solutions, and also in the washing of carbonate of sodium from black ash. A long box, rectangular in section, was divided into compartments which allowed the solution to flow upwards through the cake placed on a filter frame, and downwards between them. Filter cloths used both above and below the cake consisted of cloth woven one way, and hair the other; flannel or felt may be used also—or even calico. Solids were thus retained between the upper and lower cloths in each compartment, while the soluble salts were removed with a current of water. In this way washing can be made continuous and automatic; the solutions escaping would also have the maximum quantity of silver dissolved if the flow were properly regulated. The washing box could also be kept locked. The escaping solutions containing the silver could be precipitated on copper in similar boxes, or if copper and other base metals were absent from the solution the precipitation could be done on iron. If copper is present in solution the silver can be thrown down on copper, and the copper afterwards precipitated on scrap iron; the copper precipitate being available for the precipitation of the silver. The main trouble about the continuous washing of the fused cake, lies in the fact that a portion of the gold is in a fine state of division, and the presence of a comparatively large amount of fine insoluble compounds, such as silica, sulphates of calcium and lead. The method described for the removal of calcium and lead sulphates might be made use of in the box, but the solutions from the sodium carbonate, and from the nitric acid, should be run into separate sumps, and not be allowed to pass in with the silver solution.
Treatment of the Insoluble Residue
When the material left after washing out the soluble salts is dried and smelted direct a very small amount of flux is necessary; for instance, in some cases silica is the only insoluble material, when about one and a half times its weight of carbonate of sodium will be all that is required to flux it. This may be carried out in an ordinary plumbago crucible.
When lead sulphate is present the smelting cannot be made in a plumbago crucible without some of the lead becoming reduced and passing into the gold. If a fireclay pot is used and reducing agents excluded, the silica present in the auriferous residue will combine with lead oxide, and sulphur tri-oxide is evolved.
PbSO + SiO2=PbSiO3 + SO3
Calcium sulphate is also decomposed to a certain extent in a similar manner, but when these insoluble sulphates are present it is better to use about their own weight of borax glass to obtain a fluid slag. The addition of a small quantity of fluorspar is of much assistance in promoting the fluidity of the slag when calcium sulphate is present.
Amalgamation of Gold Slimes during the Refining Process
The objection to dealing with finely divided gold in smelting pots has been already mentioned. To avoid the possibility of loss in that direction, also the necessity of making slags, the separated gold can be collected by amalgamation, and the admixture mechanically washed off. When the gold is all coarse and spongy this may be readily done, but it is a difficult matter to amalgamate the finely divided gold, especially that present as a pink powder. Amalgamation must be performed in such a way as to bring the gold into contact with the mercury, so that it will amalgamate. If mercury is poured on the mass it sinks through it, and very little amalgamates, but if the mercury is squeezed through a cloth so as to become finely divided when introduced, and if the pulp is stiff enough to subdivide globules of mercury when all are ground together the gold will amalgamate readily. The usual compounds used to keep the mercury bright and clean may be added, but it will be found that cyanide of potassium is one of the best agents for the purpose. The cyanide added will also dissolve any very fine gold which does not readily amalgamate, so that at the close of the operation only gold in amalgam, and some gold in solution as double cyanide, will remain. As soon as this stage is reached the sand and such like impurities can be eliminated by washing them off into a vat, the sediment may be allowed to settle, and the gold can be precipitated in a small zinc box, the cyanide solution being returned to the mill solutions. The gold can also be thrown down as a cyanide by the addition of acid in sufficient quantity to neutralise any free potassium cyanide present, or as metallic gold by the addition of scrap zinc, and acidifying the solution. The insoluble mud, after decanting the clear solutions containing the gold, can be washed free from gold, or simply allowed to accumulate, and then added to the agitators, or to the vats, when the small amount of gold it may contain in a soluble state enters into circulation with the rest of the solutions.
Effect of Sulphate of Lead
When sulphate of lead remains with the gold residues it is found that lead enters the gold amalgam, and will be found in the retorted gold. If it is the only impurity present it may be got rid of by methods already described. The method of treatment by amalgamation offers several advantages, and despite the fact that one step more, retorting, is added to the process, the smelting of retorted gold is a simple matter compared with the smelting of gold and slagging off a large amount of impurities.
Proportion of Coarse to Fine Gold
It was recognised that if only coarse gold could be formed that the whole process would be rendered much simpler. In order to find out the amount of each present some experiments were made, and the following result is typical:
Slimes obtained from the N. Kalgurli Mine, W.A.
Slimes, 10.0 grms.
Sulphuric Acid, 1.5 grms.
Water sufficient to moisten.
After the heating almost to dryness, 10grms. of nitre cake were added, and the whole heated until the pyrosulphate had decomposed. The silver sulphate was washed out with water, and the fine slimes allowed to overflow into another vessel. The amounts left were as follow:
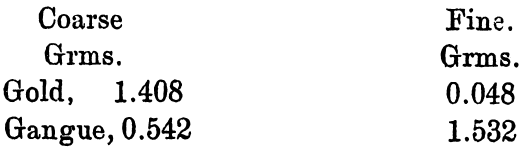
Or about 3 per cent, of the gold was finely divided.
Attempts were made to see if the production of fine gold could not be avoided. It invariably forms when such substances as calcium sulphate and silica, which are insoluble in fused pyrosulphate of sodium, are present. The gold adhering to such substances did not appear to have gone through a transition stage such as that which came together in spongy masses. In order to see if on adding some solvents the gold could not first be dissolved, and then be gathered together, the following tests were made:
Slimes…………………………………………………………………………………..5 grammes.
Nitre……………………………………………………………………………………..1 gramme.
Salt………………………………………………………………………………………2 grammes.
Sulphuric Acid……………………………………………………………………….4 grammes.
The first three were mixed and heated, then cooled, the sulphuric acid was then added, hydrochloric and nitric acids were evolved. The mass was then heated strongly. A bright coherent film of gold appeared on the surface of the melt. On adding water it was found that decomposition was incomplete. The sulphuric did not penetrate the whole mass. The silver chloride which formed was removed by ammonia, the gold left assayed 9701, but was not free from the pink powder.
Next a charge of the following materials was taken:
Slimes…………………………………………………………………………………….5 grammes.
Nitre……………………………………………………………………………………….1 gramme.
Salt…………………………………………………………………………………………1 gramme.
One gramme of sulphuric acid was placed in the bottom of the crucible and the mixture placed above. The mass was gently, then strongly, heated until it fritted. Chlorine was freely evolved. Three c.c. of sulphuric acid were then added. The sulphuric acid, on heating, appeared to volatilize without causing the gold to run together. Five grammes of nitre cake were added, and the whole fused. On washing no pink powder was visible, but the bottom of the porcelain crucible was found to be stained pink. The gold obtained assayed 9865, but the loss by volatilization amounted to nearly 10 per cent. Nitre was excluded, and a charge made up as follows:
Slimes…………………………………………………………………………………….5 grammes.
Salt…………………………………………………………………………………………1 gramme.
The crucible was charged with 1 grm. sulphuric acid, and the slimes and salt added. On gently heating, the evolution of hydrochloric, hydrocyanic and organic volatile products took place. The mass sintered and was uniformly brown on cooling; 3 c.c. of sulphuric were then added, and the whole gently then strongly heated. On cooling the mass did not alter in color. It remained brown, with no gathering together of the gold: 5grms, of nitre cake were then added, and the whole fused. The fuse was porous and dark in colour, and although it did not contain any pink powder yet was not wholly decomposed, since chloride of silver formed when water was added. The gold obtained assayed 9243, and slightly over 10 per cent, was lost by volatilization. It is, therefore, inadvisable to use salt in the fuse, since gold may be transformed to a chloride and volatilised; it is also difficult to decompose the whole of the chlorine compounds unless a very large excess of sulphuric acid or nitre cake is used. While these methods get rid of the pink powder it is probable that it is the fine gold which first volatilises. It should, however, be noted that by using a very high temperature with nitre cake alone, so as to decompose part of the sulphate of silver formed the pink powder disappears.
Experimental Work on Various Gold Slimes
In carrying out a small test at the Waihi Gold Mines, N.Z., on slimes which carry about 4 parts of silver to 1 of gold, the author obtained a gold button which assayed 996.7. Further tests were made by Mr. Hubert W. Hopkins, A.R.S.M., and the following details were kindly supplied by Mr. Geo. G. Banks, the metallurgist to the company:—
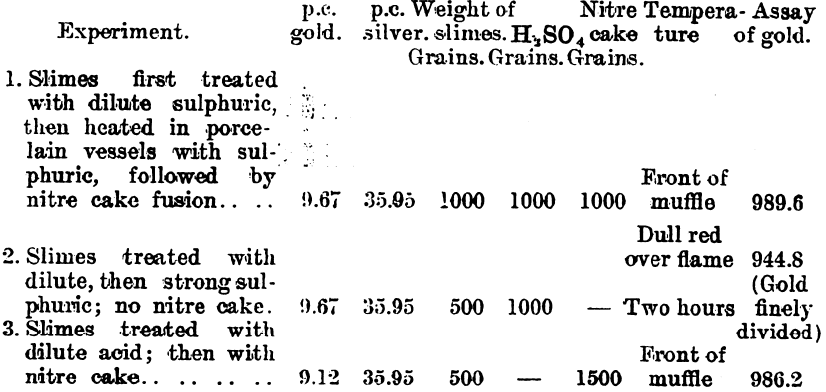
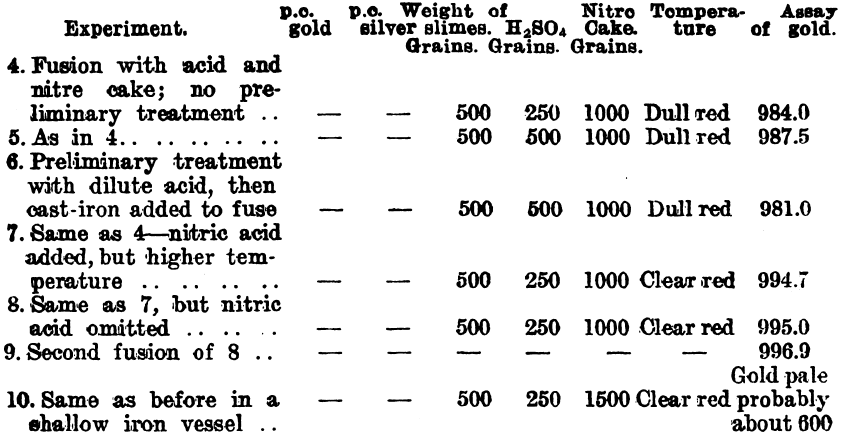
Since it was found that a large surface of iron had a reducing effect upon the silver sulphate on fusion, experiments were conducted on a working scale to find what result could be obtained. These experiments were made on a dried slimes after a preliminary treatment with 20 per cent, sulphuric acid. The washing of the fused product was done in a pointed wooden box.
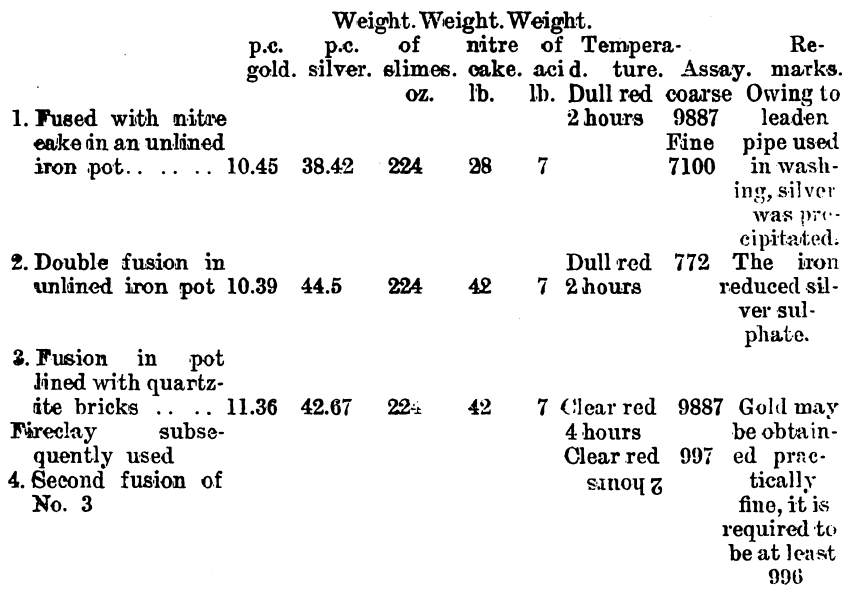
Although iron pots may be used when the amount of silver is very small, and, therefore, is not brought into contact with the iron to an appreciable extent, yet when a large amount of silver is present, and the molten sulphate is brought into contact with iron, the silver is reduced. Experiments made by myself in Australia and in Mr. Abbot Hank’s laboratory, San Francisco, by Mr. Robert Allen, M.A., have shown this to be the case. On lining the iron vessels with fireclay, and using a solution of water glass to moisten the clay, then drying and slowly baking, the vessels were found to stand admirably. When the clay is not cemented by the silicate of soda it is apt to disintegrate on fusion, and mix with the gold, but when treated as described a coherent glaze is obtained.
Tests made on Western Australian gold slimes gave uniformly good results in the laboratory. Samples from the Great Boulder mine were treated with one-fifth of their weight of sulphuric acid, and from one-third to three times their weight of nitre cake, gave in each case coherent cakes of gold. The larger the quantity of nitre cake the more easily was the washing performed. A test was made in an iron pot using slimes, 50 oz.; sulphuric acid, 68 per cent., 200 c.c.; nitre cake, 100 oz. The slimes were first mixed with sulphuric and a few ounces of nitre cake, then heated to dull redness, after which the rest of the nitre cake was added. The time occupied by the drying and fusion was 30 minutes. The resulting fused product was washed as well as could be done by decantation, as no large filtering appliances were available at the time. There was still silver present as sulphate, but the residue was taken, dried and smelted, and assayd 987.3. Laboratory tests on the same material gave gold 997 fir e. At the Great Boulder Perseverance mine the following analysis was made by Mr. H. B. Wright:

A laboratory test on these slimes yielded a comparatively large amount of pink, powder, mainly due to the insoluble salts and substances present. Subsequent tests of this pink powder proved it to be composed of gold and silica, no silver remaining.
At the Ivanhoe mine the gold slimes when first subjected to the dilute acid treatment, and the resulting product, after being washed with water, smelted, prouce a base bullion assaying approximately:

Experiments were made from the raw slimes from this mine, 10 grms. raw slimes were treated with 2 cc of sulphuric acid, and water sufficient to moisten. This was dried and heated; 15 grammes of nitre cake were then added, and the mixture heated for 10 minutes in the muffle. The gold ran together well. On assaying duplicate samples, their assay values were respectively:
(a) 996.2 (b) 996.3
Similar slimes were taken, and first subjected to a dilute acid treatment in order to remove the zinc as sulphate. These slimes were moistened with sulphuric acid as before, and then mixed with an equal weight of nitre cake, and fused as before; the gold from the samples so treated assayed as follows:
(a) 990.5 (b) 992.4
The Oroya-Brownhill Company produces bullion rich in tellurium. During treatment of the ore a solution is made up to carry 0.1 per cent, of cyanide of potassium, and cyanogen bromide is afterwards added to the extent of one pound for each ounce of gold present in the ore under treatment. The ore is agitated with the solution for 12 hours. During this time the whole of the cyanogen bromide disappears. The raw ore, on which this solution is used, contains telluride of mercury, silver, and gold. These dissolve in the solution, and are precipitated in the zinc boxes. On cleaning up the slimes are first subjected to a dilute acid treatment, to remove the zinc; they are then roasted on iron trays in a cast-iron muffle, in which part of the tellurium and the whole of the mercury are expelled, and the base metals partly oxidised. The precipitate is then smelted, using 50 borax, 20 sand, and a little soda to every 100 parts of the roasted slime. The bullion produced is very base, assaying only 730 fine. It is brought up to 900 fine, and most of the tellurium is eliminated by granulating it and treating it with nitric acid. The granules are placed in an earthenware jar standing on a sand bath; water is added to cover them, and then nitric acid is added. Heat is applied for 9 hours. The solution is then poured off, and more acid applied. After washing with water the granules are sprinkled with nitre and heated in a muffle for about two hours, and finally smelted with the addition of borax, glass., sand, and a small amount of manganese dioxide. During the washing tellurious acid separates out copiously as a heavy white powder. Some explanation of this method of refining has been already given.
On trying some experiments on raw slimes from this mine with sulphuric acid, followed by nitre cake, the gold ran together well, and although tellurious acid separated out on washing, it could be removed mechanically, or dissolved up in a small quantity of acid, after the soluble salts of silver and sodium had been removed.
Experiments made at the South Kalgurli, Associated, and other mines at the Boulder, showed that by this method of treatment it would be possible to obtain fine gold from their gold slimes.
