In simplified terms, the Effect of Oxygen on Gold Leaching can be summarized in saying: The dissolution rate of gold in alkaline cyanide solutions under atmospheric conditions, and at practical cyanide levels, is directly proportional to the dissolved oxygen concentration.
The rate of dissolution of gold in alkaline cyanide solution is dependent on a number of factors, principally the solution composition (pH, complexant concentration) , the redox potential (oxidant concentration), the surface area of the gold, temperature and mass transport rates (mixing).
The ores invariably are high cyanide consumers and not surprisingly they usually are excessive consumers of oxygen. Undoubtedly some leach problems have been tackled by adding excessive amounts of cyanide when the underlying problem was a lack of dissolved oxygen in the leach solutions.
In a practical situation, several of these factors can be ignored since they are either constants or we have no direct control over them:
1) Temperature: Under atmospheric leaching conditions there is little that can be done to control the temperature – we will assume that it is a constant for the purposes of these calculations.
2) Surface area of gold: This is a function of the mineralogy in the case of heap/dump ores. For milled ores the surface area will be a function of the mineralogy but may also be affected by the grinding process (actual grinding of gold particles). This effect is approximately constant for grinding to a particular liberation size and can be ignored.
3) EH: There is a narrow operational pH band within which we need to operate. The pH conditions for gold dissolution to occur the Eh-pH diagram for the gold-cyanide aqueous system. The area above the O2/H2O line represents the Eh-pH range under which oxygen reduction can occur (and corresponding oxidation of gold).
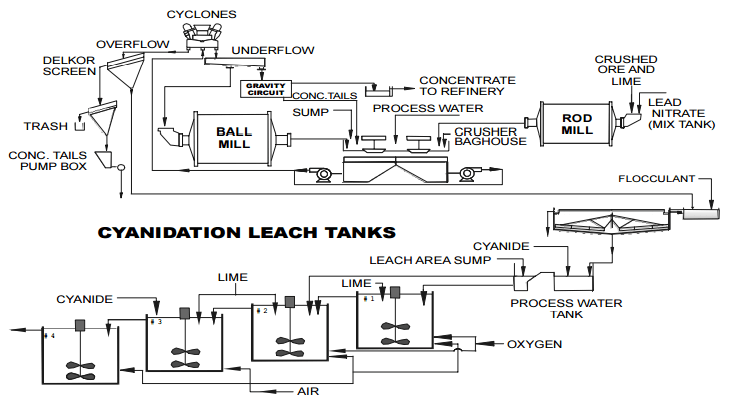
Cyanidation of a pyrrhotite-bearing gold ore
Author of The Chemistry of Gold Extraction, Second Edition and also the article iFramed below.