Table of Contents
In recent years, hydrometallurgical processes for recovery of metal values from low-grade ore minerals have played an increasingly important role. Dump leaching of low-grade copper waste materials in the western United States now yields 11.5% of the total U. S. copper production. Although scavenger operations in uranium leaching have been successful, the state-of-the-art of in-situ uranium leaching has not progressed to that of copper leaching. In the leaching of copper sulfide minerals and pyrite-bearing uranium ores the bacteria, Thiobacillus ferrooxidans and Thiobacillus thiooxidans, are commercially exploited to oxidize iron and sulfur. The importance of T. ferrooxidans in leaching operations is known to be immense as these organisms provide continual regeneration of the oxidizing agent, ferric iron, and may directly attack the mineral lattice. T. thiooxidans are believed responsible for oxidation of sulfur resulting from ferric iron reactions with metallic sulfides and other oxidation reactions. In some leaching operations T. ferrooxidans are unable to rapidly regenerate ferric iron so oxidizing agents may be necessary. It has been suggested that hydrogen peroxide (H2O2) could be added to influent leach liquors. The proposed reactions for the oxidation of ferrous iron by H2O2 are :
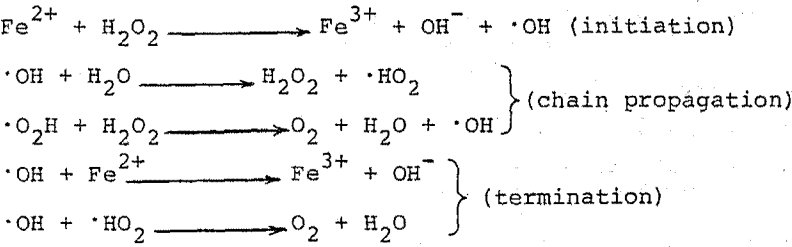
H2O2 is also used in dissolution of uranium to produce an oxidant, ferric iron, in acid leaches and to oxidize tetravalent uranium. The addition of H2O2 is intended to accelerate the oxidation of iron and reduced minerals and not necessarily to replace existing bacterial activity.
The purpose of this study is to examine the effect of on the activity of T. thiooxidans and T. ferrooxidans and investigate methods of reducing observed detrimental effects.
Experimental
The Organisms
The bacteria used in this study were Thiobacillus ferrooxidans (American Type Culture Collection 19859) and Thiobacillus thiooxidans (ATCC 8085).
Assays
Protein was measured according to the method of Lowry, et al. The colorimetric method of Grat-Cabanac was used to determine concentrations of ferrous iron.
Culturing Methods
The medium described by Bryner and Anderson was used. Elemental sulfur (flowers of sulfur) was sterilized in 0.1-g quantities by intermittent steaming for 30 min on three consecutive days. These aliquots were added per 50-ml medium (60 mM S°) as a “sulfur medium” for T. thiooxidans. A solution of 25 g FeSO4·7H2O in 95 ml distilled water and 5 ml 1 N H2SO4 was sterilized at 18 lb pressure, 121°C for 20 min. An “iron medium” for T. ferrooxidans contained 2 ml of the sterile solution added to 50 ml of medium (36 mM Fe²+).
Fernback flasks containing 1.5 liters of medium and energy source were used as batch reactors to obtain large numbers of cells. T. thiooxidans and sulfur were sedimented by centrifugation from spent medium at 27,000 g and resuspended in fresh medium. Cells were separated from sulfur by centrifuging at 120 for 10 min to sediment the sulfur. T. ferrooxidans were harvested and cleaned according to the method of Silverman and Lundgren.
Growth studies were conducted in 125 ml Erlenmeyer flasks with 50 ml medium and sulfur for T. thiooxidans or ferrous iron for T. ferrooxidans. After incubation of flasks for 7 days at 25° C0, all cultures were examined microscopically and pH measurements taken. T. thiooxidans cultures were analyzed for protein concentration, and T. ferrooxidans cultures and controls were analyzed for ferrous iron concentration.
H2O2
“Albone” M and “Albone” DG hydrogen peroxide, obtained from the Industrial Chemicals Department, E. I. duPont de Nemours & Co., assayed 35% H2O2. These solutions were diluted with medium to provide concentrations suitable for the study.
Test Methods
Manometry. Oxygen uptake of T. ferrooxidans and T. thiooxidans in concentrations of H2O2 from 0 to 10.3 mM was measured using a Gilson Differential Respirometer according to standard manometric techniques. Colloidal sulfur (160 mM) was directly added to 5 ml of medium in the reaction vessel. Two-tenths ml of acid ferrous sulfate (36 mM) were placed in one sidearm of a double sidearm reaction vessel. The other sidearm contained 0.5 ml of T. thiooxidans or T. ferrooxidans of known protein concentration or 0.5 ml of medium in control reactors. After equilibration at 25°C, the reaction was initiated by adding the sidearm contents to the reaction chamber. Oxygen uptake was followed for 1 hr, and all data were corrected for endogenous respiration.
Growth effects of H2O2. One-tenth ml of harvested T. thiooxidans or T. ferrooxidans was added to 10 ml of medium containing 0 to 7.2 mM H2O2 for T. thiooxidans and 0 to 41 mM H2O2 for T. ferrooxidans. For controls, 0.1 ml of medium was added to a corresponding series. After a 6-hr contact period, 1 ml was removed and cultured.
Effect of H2O2 in simulated leach system. These components were added to 125 Erlenmeyer flasks in the following sequence: 1) Bryner and Anderson medium, 2) concentrations of H2O2 from 0 to 15.4 mM, and 3) ferrous iron in 20% stochiometric excess of the amount oxidized by H2O2 according to the reaction:
H2O2 + 2Fe²+ + 2H+ → 2H2O + 2Fe³+
A reaction period of 15 min was followed by addition of 36 mM ferrous iron and inoculation with 0.1 ml T. ferrooxidans or 0.1 ml medium for controls. The flasks were incubated at 25°C for 7 days Adding components in the described sequence simulated the addition of H2O2 to a precipitation plant effluent. H2O2 would be totally reacted by the time the solution arrived at the oxidation pond or dump environment. The further addition of ferrous iron assured supplemental energy for T. ferrooxidans that inhabit the oxidation pond and leach area.
Results
Manometry Experiments
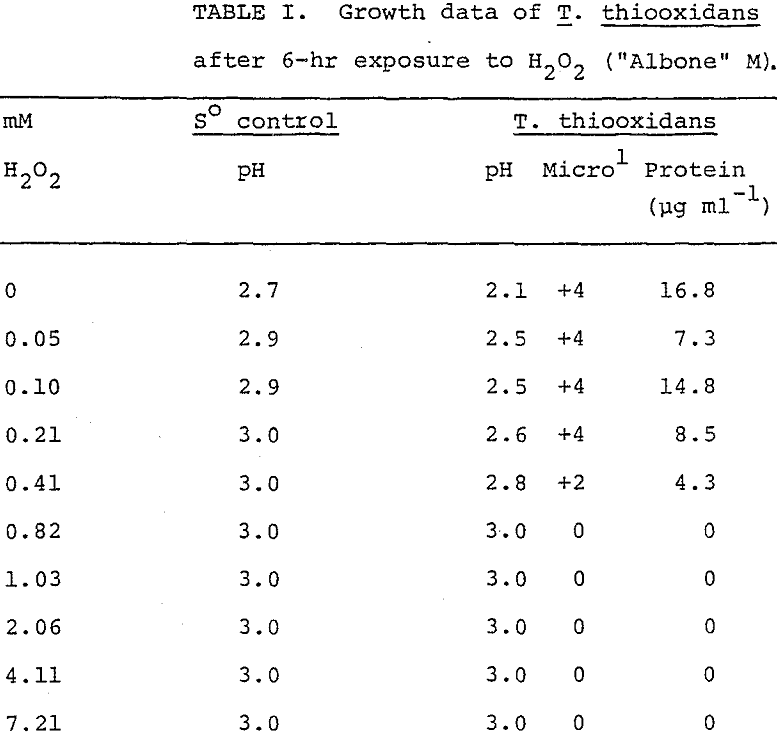
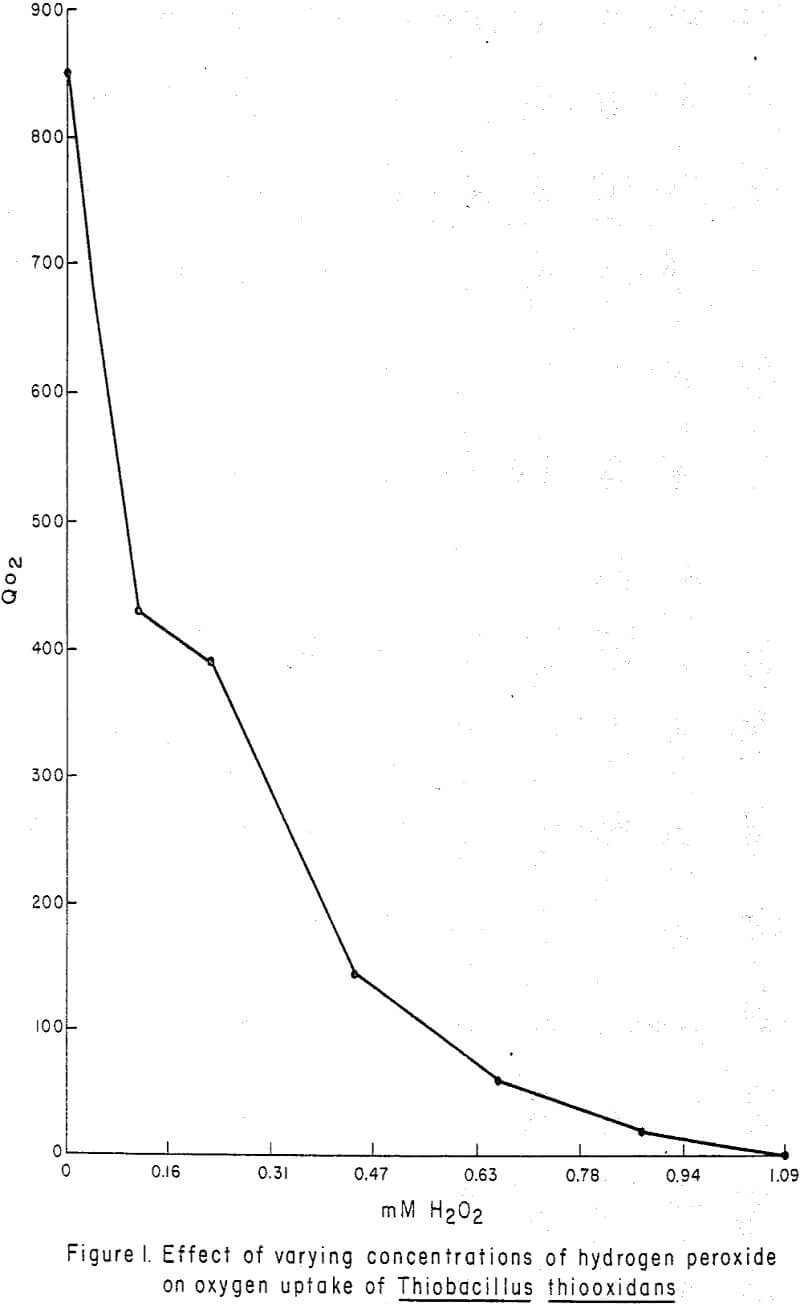
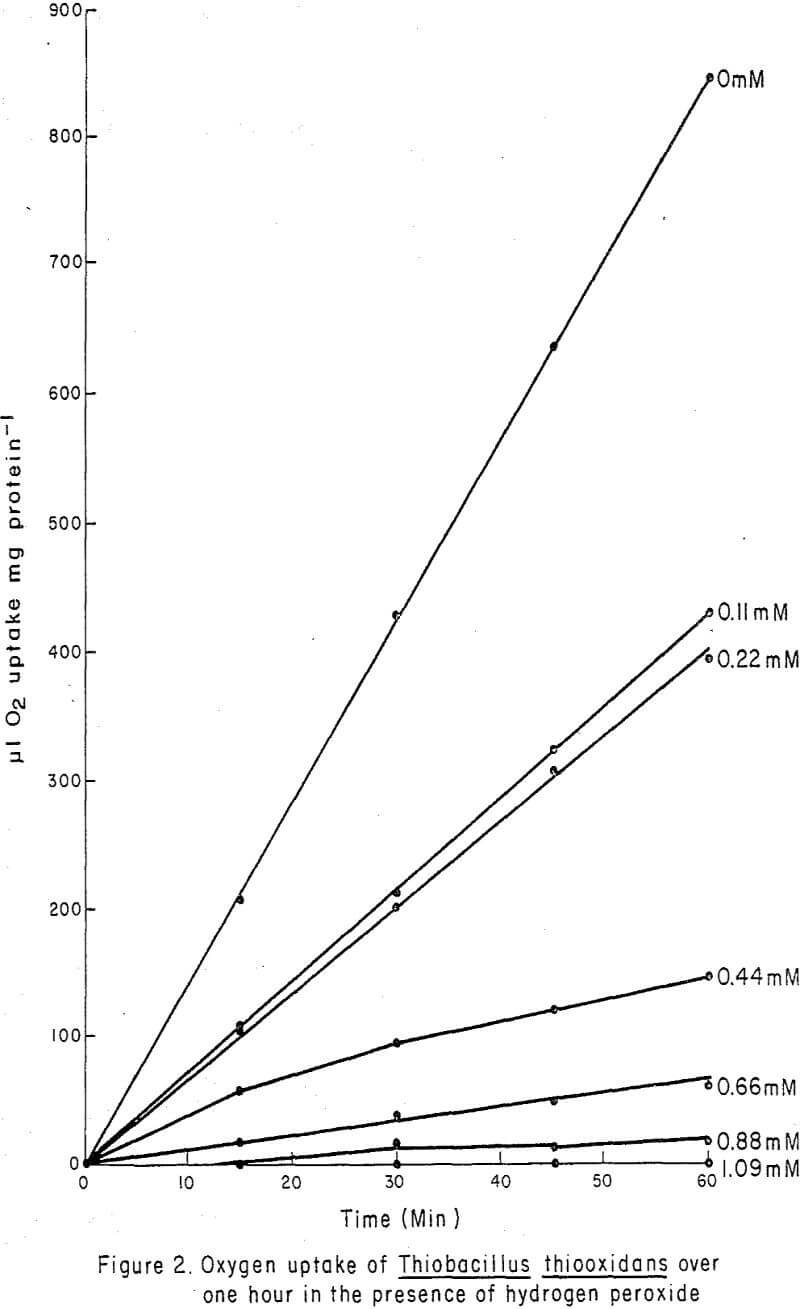
Figure 1 graphically illustrates the effect of H2O2 (“Albone” M) concentrations from 0 to 1.0 mM on the QO2 (µl O2 uptake mg-¹ protein hr-¹) of T. thiooxidans. The curve shows an asymptotic approach to of zero. Oxygen uptake was linear with time (Figure 2) and was completely inhibited at 1.0 mM H2O2.
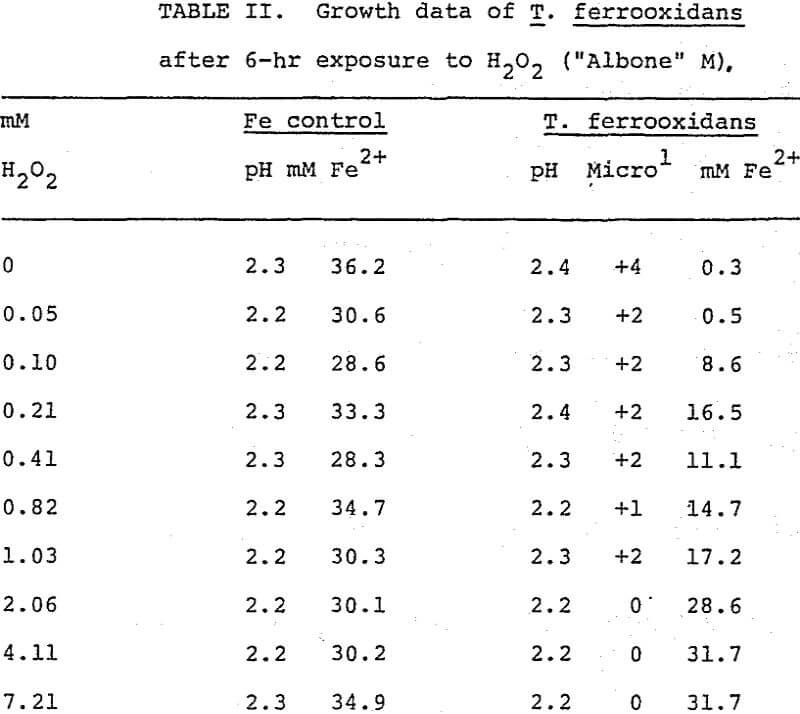
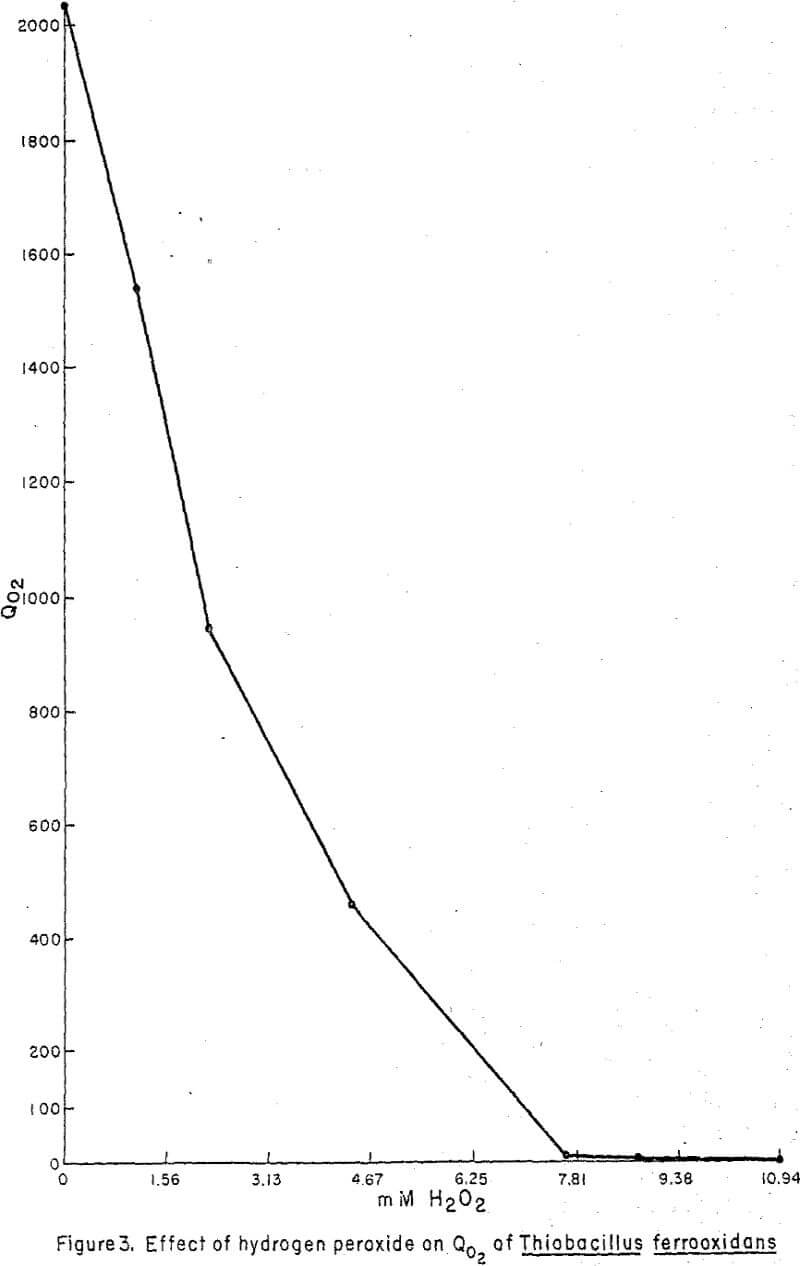
Oxygen uptake by T. ferrooxidans was slightly inhibited in the presence of low concentrations of H2O2 (“Albone” M) and inhibition was complete at 8.75 mM (Figure 3).
Growth Studies
T. thiooxidans and T. ferrooxidans were subjected to concentrations of H2O2 (“Albone” M) from 0 to 7.21 mM for periods of 6 hr to determine the effects on the growth of the organisms.
After 7 days incubation, T. thiooxidans cultures and sterile controls were examined microscopically and analyzed for protein concentrations and pH changes due to oxidation of sulfur. Table I shows data illustrative of six experiments. Growth of T. thiooxidans was inhibited after exposure to H2O2 (“Albone” M) in excess of 0.21 mM as indicated by the lack of growth and the increase in pH. Inhibition was complete at 0.82 mM H2O2. Corresponding results were observed for “Albone” DG.
Table II presents representative data collected from T. ferrooxidans cultures and uninoculated organisms. Growth was greatly curtailed at 0.10 mM H2O2, and inhibition was complete at 2.06 mM H2O2 as indicated by, the ferrous iron analyses corresponding with the uninoculated control. Similar inhibition of T. ferrooxidans was noted with “Albone” DG.
H2O2 in Simulated Leach System
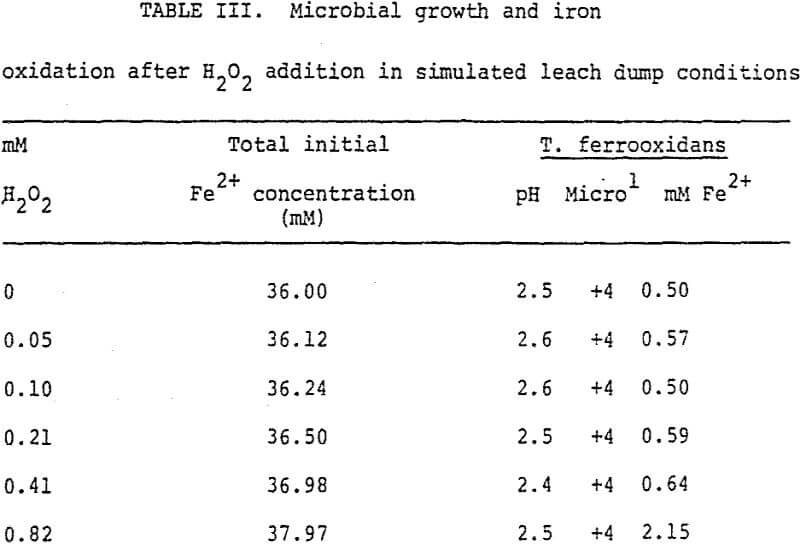
Iron was added in 20% stochiometric excess of the H2O2 (“Albone” M) present. T. ferrooxidans and 36 mM ferrous iron were then supplied. Results from these studies (Table III) showed that iron was actively oxidized at 0.82 mM H2O2. Although iron was not actively oxidized at higher H2O2 concentrations, excellent growth of T. ferrooxidans was observed at 4.11 mM H2O2. At concentrations exceeding 2.51 mM H2O2, growth was diminished. At high ferrous iron concentrations the pH was less than 2.0 due to the acidic nature of the ferrous sulfate.
Discussion
In manometric studies, low concentrations of H2O2 (0.16 mM) (Figure 1) greatly diminished oxygen uptake by T. thiooxidans and 1.09 mM completely suppressed oxygen uptake, but somewhat larger concentrations (0.41 mM (Table I) were required before growth was no longer possible. The linearity of oxygen uptake (Figure 2) demonstrated by T. thiooxidans in the presence of H2O2 suggests that the rate of oxygen uptake during the 1-hr experimental period was constant for each H2O2 concentration, but that the rate was greatly diminished at high H2O2 concentrations. The manometric study indicated that H2O2 adversely affected the ability of T. thiooxidans to oxidize sulfur. When T. thiooxidans cells were subjected to H2O2 for 6 hr and then cultured, it was established that the inhibition of substrate oxidation was due to a lethal effect of H2O2 rather than a temporary suspension in activity.
T. ferrooxidans was far more resistant than T. thiooxidans to the detrimental effects of H2O2. Oxygen uptake was slightly inhibited at an H2O2 concentration of 1.56 mM, but oxygen uptake was not stopped until H2O2 concentrations reached 8.75 mM. T. ferrooxidans continued growth after exposure up to 2.06 mM H2O2. These data indicate that the leach dump bacteria, T. ferrooxidans and T. thiooxidans are susceptible to quite low concentrations of H2O2, and that exposure will greatly reduce the viability of the organisms.
Further studies showed that it is possible to add H2O2 in a sequence which would be less detrimental to bacteria. Adding H2O2 to the solution containing reduced iron, but no bacteria, would closely resemble the actual practice of adding H2O2 to the precipitation plant effluent before it reaches the oxidation pond or leach dump. In this way iron is oxidized and the H2O2 is reacted before the solution reaches the predominant habitat of the bacteria. However, residual H2O2 would not be available for oxidation of reduced uranium or copper minerals in the leaching environment. It was shown that T. ferrooxidans could grow when 4.11 mM H2O2 had been added (Table II). Less growth was observed at higher H2O2 concentrations, but this may have been due to the microbe being unadapted to the lower pH of the medium encountered by adding excessive acidic ferrous sulfate. The concentrations of ferrous iron in leach solutions leaving copper precipitation plants can range from 18 – 161 mM. To oxidize this amount of ferrous iron would require 9 to 81 mM H2O2, or much greater H2O2 concentrations than were found to be detrimental to T. ferrooxidans. From preliminary bench scale testing it seems that H2O2 concentrations should not exceed 4 mM. However, this would only oxidize 8 mM ferrous iron, and it has been reported that the optimum ferric iron concentration is about 18 mM. It is possible that bacterial acitivity in the oxidation pond or on the dump surface could oxidize the residual ferrous iron in the leach solution.
Acknowledgements
The author would like to express her appreciation to the Industrial Chemicals Department, E. I. duPont de Nemours & Co., for the financial support of this study and their permission to publish the results.