Table of Contents
A coke Cupellation furnace still in use at the Mint is shown in front and side elevation in Figs. 95 and 96. It consists of an outer casing of wrought-iron plates about 1/8 inch thick, united by angle iron. This casing is connected with a chimney 60 feet high by means of a wrought-iron hood, a, and flue which is provided with a damper, b. The lining consists of Stourbridge firebricks. Fuel is introduced through the opening c.
The muffle, d, is made of a mixture of graphite and fireclay which lasts better than fireclay alone. Its mouth is closed by the graphite block, e, and the sliding iron plate, f, when cupellation or scorification is going on. For annealing and other purposes the sliding doors, g, g, are used. Air is admitted to the muffle above the block, e, only, and is withdrawn through the graphite tube, h, which connects with the iron tube, k. This is furnished with a damper, l, and is connected with the chimney. There is no other opening in the muffle, so that the draught through it is quite independent of the draught through the furnace. This is an important feature which should be adopted in all muffles. The muffle rests on a bed of fireclay and pieces of firebrick, which covers the cast-iron girder-plate, m. The top of the muffle is covered with a thick layer of fireclay and graphite to check radiation and to preserve the muffle. The clearance between the muffle and the sides of the furnace is 3¼ inches, but at the back it is only 1 inch, and this space is filled with fireclay to prevent overheating. In the gas furnaces the muffle now fits closely against the back wall of the furnace, so that the back is not hotter than the front.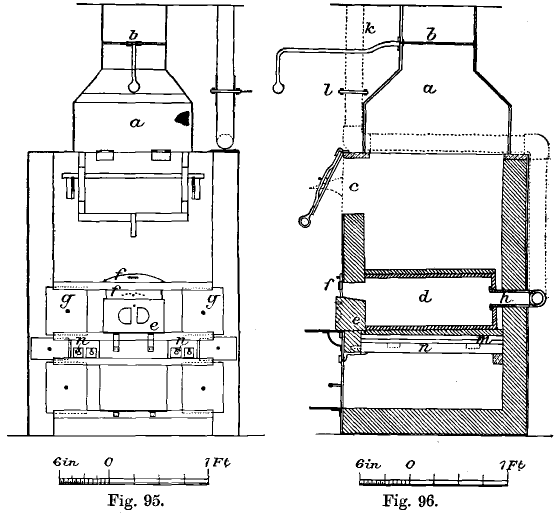
There are eleven fire-bars, n, n, but only the three outer ones on each side are covered with fuel. On the other five bars rests the cast-iron girder-plate (Fig. 97), which is flat on the upper surface, but is strengthened with ribs on the under surface in order to prevent buckling. Charcoal, anthracite, or coke can be used in this furnace.
The use of assay furnaces, heated by gas, is now general, wherever gas can be obtained. Gas furnaces, with separate draught to the muffle, as described above, are now preferred at the Mint. Petroleum furnaces are used when coal, coke, and gas are alike difficult to obtain.
Graphite muffles, though advantageous in coke muffles, do not seem to last so well as fireclay muffles in gas furnaces.
Furnaces to burn soft coal are sometimes used in places where good coke is very expensive. They differ little from coke furnaces in construction, but have less space between the muffle and the side walls. The flame of the coal is chiefly instrumental in heating the muffle, a comparatively thin bed of fuel being employed.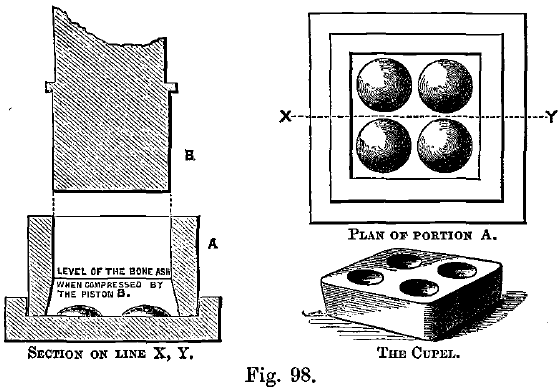
The cupels in use at the Mint are in sets of four, the outer margin being square, as shown in Fig. 98. At some assay offices, larger numbers of cupels are made in one block. The object is to facilitate charging-in and withdrawal, but the difficulty of maturing the cupels increases with the size of the block, and it is better to use cupel trays in the furnace. Single cupels can then be rapidly ranged on the tray by hand, before it is charged-in.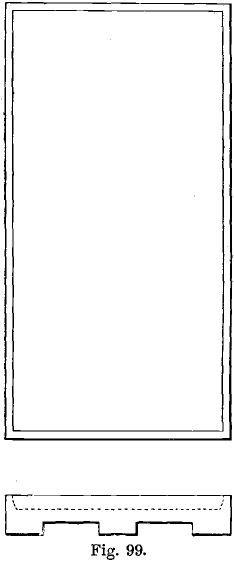
The cupel tray used at the Mint is shown in plan and section in Fig. 99. It is 11¾ inches long and 6 inches wide, and holds cupels for 72 assays, the cupels fitting tightly inside the rim. The tray is made of “ Salamander” graphite, and lasts for some weeks. Iron trays were found to interfere with cupellation and to be rapidly destroyed. Fireclay trays soon break. The tray is sprinkled with bone-ash, and the cupels placed on it before it is charged into the furnace. It is charged-in and withdrawn by an iron “peel” with two flat prongs, 1¼ inches wide, and of the same length as the tray, which slide into the grooves underneath the tray. The furnace tools formerly in use are shown in Fig. 100, where a represents the cupel tongs and b the tongs used for charging-in the lead packets. These tongs are now seldom used in bullion assaying.
Cupellation Procedure
The muffle is brought to a uniform orange- red heat before the cupellation is begun and no fresh fuel is added during the operation. The cupels are cleaned by a pair of hand bellows just before the assay pieces are charged-in. This is now done by a charging tray. It is made of nickel and consists of a plate perforated by 72 holes. Underneath is a non-perforated sliding plate. The assay pieces are put in order in the compartments, and the tray is placed in position, some help in guiding it being given by stops which touch the front cupels. The sliding plate is then withdrawn and the lead packets fall through the holes into the cupels. “Uncovering” is completed in about two minutes, and during this space of time the muffle is kept closed. The door is then opened, and the draught started by opening the damper (l, Fig, 96).
Distinct stages may be noted in the action which now takes place on the cupel. Almost immediately the surface of the molten metal becomes covered with greasy-looking drops of litharge, which are rapidly absorbed by the porous cupel and replaced by others. They pass over the surface at first slowly, but as the operation continues move with greater rapidity. In from twelve to fifteen minutes the metal suddenly becomes uniformly dull and glowing except for iridescent bands, produced by extremely thin films of fluid litharge, which are seen to pass over it. On the disappearance of these bands a bright liquid globule of a greenish tint is left, but the cupels are not withdrawn from the furnace until the expiration of another fifteen to twenty minutes so that the last traces of lead may be oxidised and absorbed. The completion of cupellation takes place first in the front rows and proceeds regularly backwards.
The cupels are withdrawn from the furnace while the assay pieces are still fluid, and “flashing” ensues in a few seconds. “Flashing” is most marked in the purer buttons, in which but little copper or lead remains. Slight effervescence may occur in these cases, but if not less than 50 parts of copper per 1,000 of gold were originally present the buttons are never sufficiently freed from base metals for “sprouting” to take place. If the cupels are withdrawn carelessly the fluid metal moves over the rough surface of the cupel, and minute beads may be separated from the main mass. To avoid this, and losses by sprouting, some assayers do not remove the assays from the furnace until the buttons have become solid.
The “flashing ” of gold assays was shown by Van Riemsdijk to be due to solidification after superfusion. The temperature of the fluid metal falls until a certain point is reached, when it solidifies, and the sudden disengagement of the latent heat of fusion reheats the cooling globule to its true melting point— viz., 950°—and a peculiarly intense light is emitted which rapidly fades as the temperature again falls. A sudden jar at any moment causes the flashing to occur instantly. If the alloy contains a minute quantity of iridium, rhodium, ruthenium, osmium or osmium-iridium (the metals of the platinum group which are non-malleable, refractory to heat, and resist the action of acids), the tendency of the cupelled metal to preserve its liquid state below the melting point, and therefore to flash during the final solidification is entirely prevented. The presence of metals of the platinum group (except platinum and palladium) in ingots of commercial gold can be detected by means of this characteristic.
The buttons (which are of the form represented at a, Fig. 102) are removed from the cupels, after cooling, by a pair of sharp-nosed pliers, cleaned by means of a stiff brush or by immersion in warm dilute hydrochloric acid, and are placed in the compartments corresponding to their cupels in the tray (d, Fig. 101). If the bone-ash is not completely removed from their lower surface it is of little moment, since bone-ash is readily dissolved by nitric acid on parting. The surface of the cupels must be carefully examined for minute beads of metal due to splitting of the lead bath, which sometimes happens if there is too strong a draught. If any such beads are found in a cupel the fact is noted and the assay repeated. If traces of lead remain in the button it is more globular, separates more easily from the bone-ash of the cupel, and has a brilliant steely surface. The effect of the presence of other metals is discussed later.
If traces of lead remain in the button it is more globular, separates more easily from the bone-ash of the cupel, and has a brilliant steely surface. The effect of the presence of other metals is discussed later.
Cupellation Temperature
The exact temperature suitable for cupellation can only be ascertained by practice, and the varying light of the day may occasion error in judging the degree of heat. The remarks on temperature in the cupellation of buttons from ores apply here. Care should be taken to ensure that the beat is so high before “charging-in” that the chilling which necessarily takes place during this operation shall not cool the muffle below the requisite temperature. It is of more consequence that the muffle should be uniformly hot throughout than that any absolute degree should be attained, as the checks used eliminate uniform errors due to high temperature.
The temperature of an assay muffle was measured by Prinsep, Assay Master of the Mint at Benares, by observing differences in the behaviour of a number of silver-gold and gold-platinum alloys when heated. He “made trials in different parts of the same (muffle) furnace. The disparity of heat,” he remarks, “is greater than might be supposed.” His results were as follows:
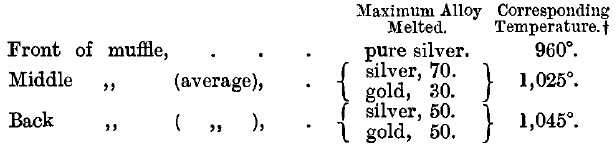
In 1892, the temperature of a muffle at the Mint was measured by the author by means of the Le Chatelier pyrometer.

The muffle was 15 inches long and 6½ inches wide, and it was found that the temperature gradually rose from about 1,050° to 1,080° in passing from front to back, whilst along the sides the temperature was 1° or 2° higher than in the middle line. The mean temperature of the muffle was about 1,070°. It is believed that with gas furnaces and the present arrangements of the muffle the variation in temperature is much less.
How to Prepare of the Assay Piece for Cupellation
If necessary, the assay piece is “flatted” on a clean anvil by a hammer with a rounded face, weighing about 11 lbs., and a portion, weighing about 0.5 gramme, is obtained by cutting with shears and filing. The metal to be cut is held firmly between the fore-finger and thumb of the left hand, and care is taken to keep the plane of the piece of gold perpendicular to the cutting faces of the shears, otherwise damage is done to the latter. Only clean portions of metal must be used.
When the assay is reported to 1/10000 part, it is evident that the balance used must clearly indicate a difference in weight of 0.1 per 1,000 or 0.05 milligramme. It is convenient to have the balance adjusted so that one subdivision on the ivory scale traversed by the pointer corresponds to this quantity. The weight of alloy must not differ from the 1,000 by more than four subdivisions of the scale, and the difference is noted down as the “ weighing-in correction.” The weighed piece is wrapped in pure lead foil together with the silver necessary for parting, and some copper unless it is present in the assay piece. The lead packets are put in order in the numbered compartments of the wooden tray shown at c, Fig. 101, p. 478, their position being noted on the assay paper. The corners of the packets are squeezed down so as to fit the cupels by pliers specially designed for the purpose, and the assays are then ready to be charged into the furnace.
It was formerly considered necessary for the metals to be present in the proportion of 1 of gold to 3 of silver, but, as early as the year 1627, Savot relates that the proportion of 1 to 2 was used, and strong acid employed in the boiling, “ quand on veut faire quelque essay curieux et exact.” Both Chaudet and Kandelhardt recommend the proportion of 1 to 2½, on the ground that less silver is then retained by the cornet than in any other case. If much more ,than three parts of silver are present the gold breaks up in the acid. Pettenkofer found that the proportion of 1 to 1.75 could be employed if the assays were boiled in concentrated nitric acid for some time. At the Royal Mint the proportion used was formerly 1 to 2.75, but is now 1 to 2 (see also p. 486 in section on “Surcharge”). The test silver should be assayed for gold, but the presence of a small quantity, say one part or less in 100,000, does not matter if pieces of the same silver are used for “proof” and ordinary assays.
The amount of lead used varies with the proportion of base metals present. For gold 900 fine and upwards, eight times its weight of lead is used at the Mint and answers very well, but the copper is not completely removed in the course of cupellation. The following table shows the proportions recommended by D’Arcet, Cumenge & Fuchs, and Kandelhardt respectively:
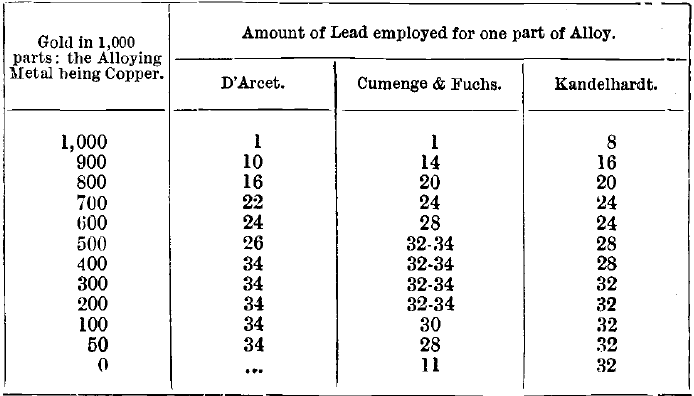
Kandelhardt’s table is modified to make it uniform with the others. It must be remembered that, although the above table gives the quantities of lead which will remove the greater part of the copper present in the alloy during cupellation, the last 2 or 3 milliemes are obstinately retained by the gold, and cannot be entirely eliminated even by a second cupellation with fresh lead. Instead of attempting to remove all the copper present in an alloy of low standard by one operation, using large quantities of lead as above, it saves time and gives more uniform results if a smaller amount of lead is used in two successive cupellations. For one part of gold bullion 400 fine, 16 parts of lead are enough if added in this way. The object in view is not to remove all the copper by cupellation, but to obtain a well-formed, clean and bright button suitable for parting. Some copper must be retained by the assay piece in order to prevent “ sprouting ” at the moment of solidification of the cupelled button. For this reason, the amount of copper in the assay piece charged into the furnace should be not less than 50 parts per 1,000 of gold.
The cupellation of lead for gold differs very little from that of lead carrying silver. When the gold is accompanied by a larger proportion of silver, and both have to be determined, the cupellation must be conducted exactly as in a silver assay, the usual precautions being taken to moderate the temperature so as to lessen the cupellation loss and to promote a slow and undisturbed solidification in order to avoid spirting. If, however, the gold predominates the finish should be effected at a higher heat, as the melting point of gold is 100° higher than that of silver. The bad effect of a higher temperature in increasing the cupellation loss need hardly be considered in the case of such small buttons of gold as are obtained in assaying gold ores, as any loss there may be is hardly appreciable by the balance. With larger quantities of gold, however (as in assaying gold bullion), this loss becomes important; and it is therefore necessary to very carefully regulate the temperature of the muffle so as to minimise the loss.
The cupels are made of well-burnt bone-ash, of the fineness of coarse wheat flour, moistened with one-twelfth its weight of water and compressed into shape in suitable moulds. The moulds sold for this purpose are often of unsuitable shape. Since lead has a specific gravity of over 11, a cup to hold from 15 to 25 grams of molten lead need not have a capacity of more than about 2 c.c. A hollow about 1 inch across and ¼ inch deep is sufficient; and the body of the cupel to absorb this weight of lead should itself weigh from 20 to 25 grams. The button of lead in a gold assay may be twice as heavy as this. For these larger buttons a hollow 1 1/3 inch across and 1/3 inch deep will be sufficient. If these larger cupels are not at hand the larger buttons will have to be reduced in size by a scorification before cupelling. In some cases this preliminary scorification is advantageous or even necessary: this may be because the lead is hard and impure, or it may be that a very small button of gold is expected. In the latter case it is best to scorify the lead down to something less than 1 gram, and to perform the cupellation on a specially prepared small fine cupel. These small cupels are best made by grinding the unsaturated portion of a used cupel to a fine powder, and compressing the dry powder into a small Berlin crucible or scorifier; the face should be made quite smooth by pressure from a pestle. On such cupels a small speck of gold (less than .01 milligram) will be left in a good shape and easily visible; but the cupel must be withdrawn from the muffle as soon as the cupellation is finished to make sure of always getting the button in good condition. In places, such as Mints, where large numbers of bullion assays are regularly made a special form of cupel is used so that not less than six dozen assays may all be cupelled at the same time in a muffle of ordinary size. These cupels are square blocks, a little less than 2 inches across, and a little more than three quarters of an inch deep. Each block carries four hollows of about .7 inch across and .3 inch deep. A muffle, on a floor space of 6 inches by 12, would take 3 of these blocks abreast and 6 deep, and thus provide the means for 72 assays.
Cupels made with wet bone-ash should be slowly dried; and if in the muffle they can be slowly brought to an orange-red heat it is all the better. Under no circumstances must the lead be placed on the cupel before the latter has been so thoroughly heated that it can no longer give off steam or gas of any kind. For this gas bubbling through the molten metal spatters it, thus spoiling one assay and throwing doubt on all the rest. Again, the risk of freezing at the start is much greater with a cupel which has not been properly heated.
The best plan is to do all the cupellations in batches. After the muffle has cooled down for the withdrawal of the last batch, and the old cupels have been taken out, the new cupels for the next batch should be put in their place. The furnace should then be stoked and made ready for the next cupellations; by the time the furnace is ready the cupels will be ready also. There should be no unnecessary handling of the cupels once they have been placed in the muffle.
The cupellation temperature for gold is an orange-red heat or perhaps a little hotter. Beginners, who are apt to overheat their furnace, should avoid a heat which can properly be called yellow. Dr. T. K. Rose has determined the temperature of a muffle during the cupellation of gold-silver alloys at the Royal Mint. In one muffle the temperature ranged from 1065° to 1095 ° C.; the lower temperature was of course in the front of the muffle. In another it ranged from 1022° to 1062°, and here the muffle appeared to the eye “ decidedly cooler than usual.” The alloy left after cupelling was made up of 1 part of gold to 2½ parts of silver, and was fused at 952° ; hence the usual temperature of cupellation was, say, 120° or 130° above the melting point of the residual metal. To obtain some real knowledge as to the meaning of these figures, the student should prepare pointed pieces of the following metals : silver, which melts at 945° ; gold, which melts at 1035° ; and an alloy, half silver, half gold, which melts at 990°. These should be placed on clean cupels in a muffle almost entirely closed; the temperature should be very slowly raised, and the appearance of the muffle when each metal begins to melt should be carefully noted. The cupelling temperature in Dr. Rose’s experiment was as much above the melting-point of gold as this is above that of the silver-gold alloy. The finish of the cupellation of gold or gold-silver alloys is practically the same as with pure silver ; there is the same thinning out of the litharge into a luminous film which becomes iridescent before the brightening. But the danger of spirting decreases as the proportion of gold becomes greater, and disappears when the gold is much over 30 per cent. Nevertheless it is well to let such buttons become solid undisturbed and protected from draughts in the body of the muffle. This means closing the muffle and allowing the furnace to cool down somewhat before withdrawing the cupels. Buttons solidified in this way are more malleable than when they are withdrawn promptly on the finish of the cupellation. This is important with large buttons, as in a bullion assay. On the other hand, very small buttons, especially such as have to be measured rather than weighed, should be withdrawn as soon as the luminous film has disappeared. For when this is done the button can be loosened from the cupel by merely touching it with the point of a pin, and is then safely and easily transferred to a watch glass by touching it with the head of a pin which has been moistened. It adheres to this, and if the pin is not too wet comes off at once on touching the glass, or in any case will do so on gentle warming.
Molten gold, with little or no silver, has a peculiar colour which is easy to recognise; it is more globular than a button of silver of the same size would be, and it shows less adhesion to the cupel. Just after becoming solid it glows beautifully, and this is so marked that it is a valuable help in finding the position of a button when it is more than ordinarily minute.
If the button left from cupellation is yellow it is at least half gold, and a rough guess as to the proportion of gold may be made from its yellowness; the rest of the metal is generally silver. The presence of platinum or one of the platinum group of metals makes the surface of the button dull and crystalline. The native alloy of osmium and iridium does not alloy with gold, however, but falls to the bottom of the molten metal. It shows itself in the subsequent parting as a black spot or streak on the under surface.
The buttons are removed from the cupel with a pair of pliers and then brushed to remove adherent litharge and bone-ash. Some assayers advise cleaning by dipping in warm dilute hydrochloric acid followed by washing in water and drying. The button is next weighed. When the quantity of silver obtained is not required to be known the weighing may sometimes be omitted. The next operation in either case is parting either with or without a previous inquartation.
The loss of gold in cupellation is by no means always inconsiderable. In three cupellations of 1 gram of gold with 20 grams of lead made purposely at a very high temperature the cupel absorbed 6.04, 6.20, and 6.45 milligrams of gold. Hence at a high temperature there may easily be a loss of more than half a per cent, of the gold. In ten cupellations with the same quantities of gold and lead, but at an ordinary temperature, the gold recovered from the cupels varied from 1.37 to 1.92 milligrams, and gave an average of 1.59 milligrams. In round numbers the cupellation loss of pure gold is .15 per cent.
But if the gold be alloyed with silver the loss is diminished, as is shown by the following experiments. Gold, .3 gram, was cupelled with 10 grams of lead and varying amounts of silver, and the cupels were assayed for gold with the following results:
These, calculated on the .3 gram of gold, give the loss as .157, .107 and .057 per cent, respectively. The effect of copper, on the other hand, is to increase the cupellation loss, which, silver being absent, may from this cause rise to .3 per cent., even when the temperature is not excessive.
In the ordinary assay of gold-copper alloys a constant weight of the alloy is always taken ; hence as the weight of copper in a cupel charge increases, the weight of gold decreases. The silver, on the other hand, is always very nearly two and a half times as much as the gold, whatever its quantity may be. But the cupellation loss is smaller with less gold and greater with more copper, and it so happens in these assays that these two opposites nearly neutralise-one another. Mr. W. F. Lowe found the gold recoverable from the cupels 0n which 20 grains of gold bullion had been treated varied only between .014 and .015 grain (i.e. from .07 to .075 per cent, of the bullion treated), although the quality of the bullion varied from 9 to 22 carat, But in the poorest bullion there was only 7.5 grains of pure gold, while in the richest there were 18.3 grains; yet each lost on the cupel the same weight of gold, viz., .014 grain. When reckoned in percentages of the actual gold present the losses are .187 per cent, and .076 per cent, respectively. The heavier percentage loss is mainly due to the increased quantity of copper.
As with silver so with gold the predominant cause of the cupellation loss is the solution of the metal in the molten litharge which passes into the cupel. Three lots of 1 gram of gold cupelled each with 20 grams of lead repeatedly, so as to make 13 cupellations in all, lost in actual weight 35.72 milligrams. The gold recovered from the cupels amounted altogether to 34.56 milligrams. This shows that, compared with the absorption by the cupel, the other causes of loss are inconsiderable.
The loss of gold by volatilisation is, however, a real one. The dust from the flues of assay furnaces has been tested on several occasions and found to contain gold, though in small quantity. Thus Mr. Lowe found .073 per cent, of silver and .00033 Per cent, of gold in such a material. The lead volatilised from a gold bullion assay would need to be ten times as rich as this to account for a loss of gold equal to the hundredth part of a milligram. Dr. Bose, in the paper already quoted, believes that on a .5 gram charge of standard bullion the loss from volatilisation is not less than .025 nor more than .05 milligram of gold.
By way of conclusion it may be said that the cupellation loss of gold is about .07 per cent., and that it is largely met or even over corrected by a compensating error due to silver retained in the gold after parting.
