Table of Contents
Many of the major copper ore bodies throughout the world contain chalcocite (79.9% Cu) as the principal copper mineral, yet the grades of copper concentrates produced from these ores rarely exceeds 25 to 28% Cu and in one known instance went as low as 13%. The low grade has traditionally been attributed to the presence of “activated pyrite” and/or chalcopyrite, and the universal approach has been excessive regrinding with all of its inherent effects-high capital, energy and maintenance costs, excessive sliming, and concentrate filtration difficulties (high moisture content).
Rimmed pyrite particles at a minus 65 mesh grind are usually observed in two forms:
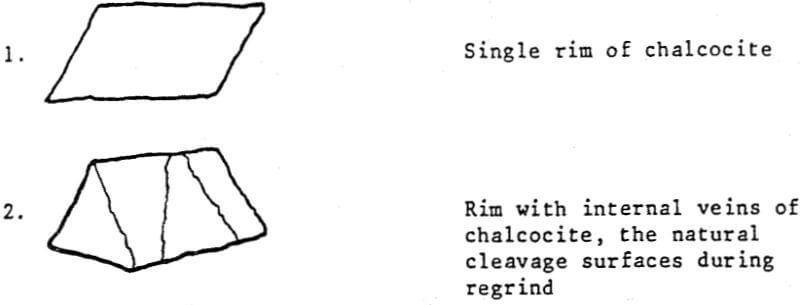
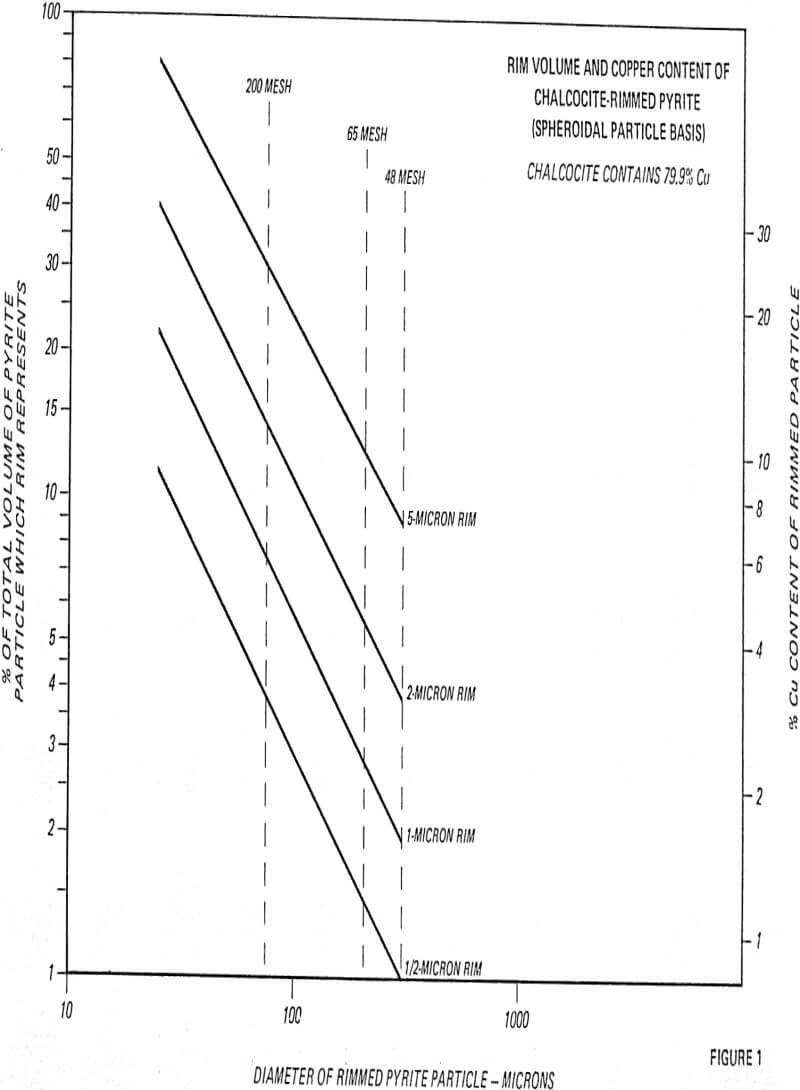
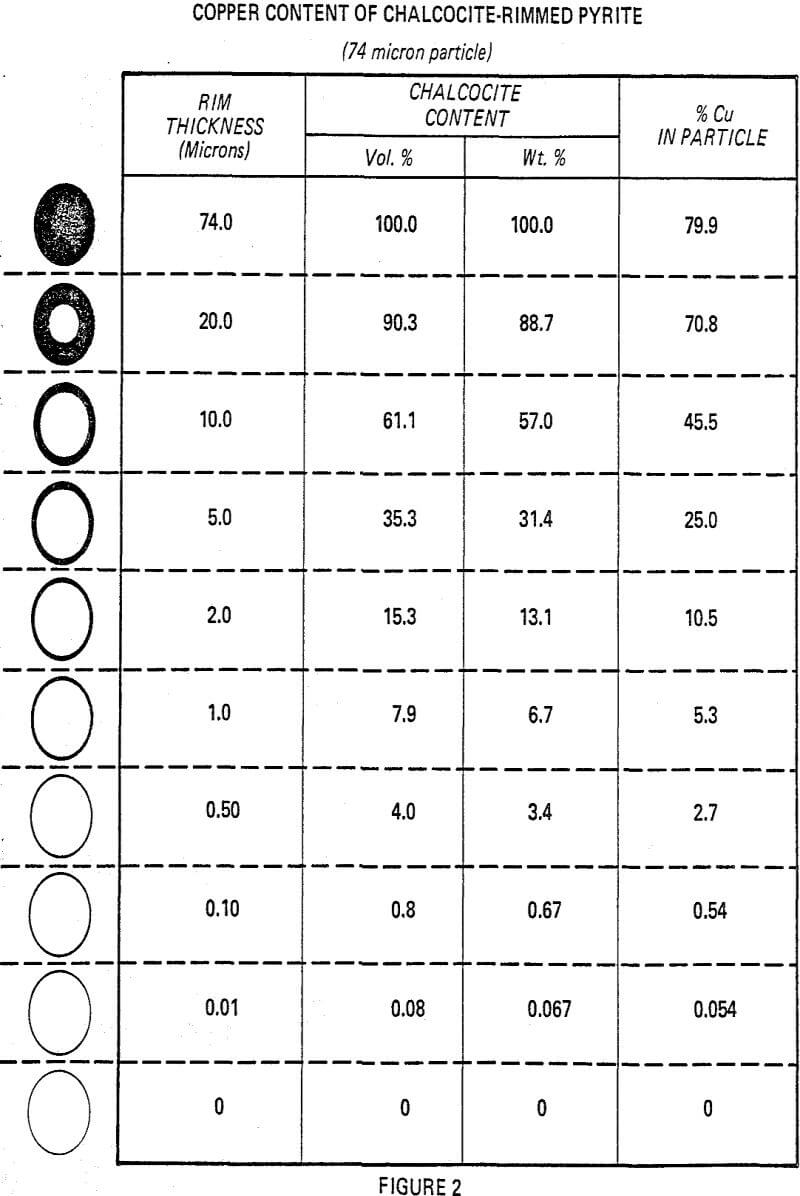
In many instances, these rims are one micron or less, with mono or dimolecular films being invisible at 400 to 600 magnification. Thus, the rims responsible for major lowering of grade represent a very small fraction of the total copper content (1 to 3%).
Summary of American Cyanamid Investigations
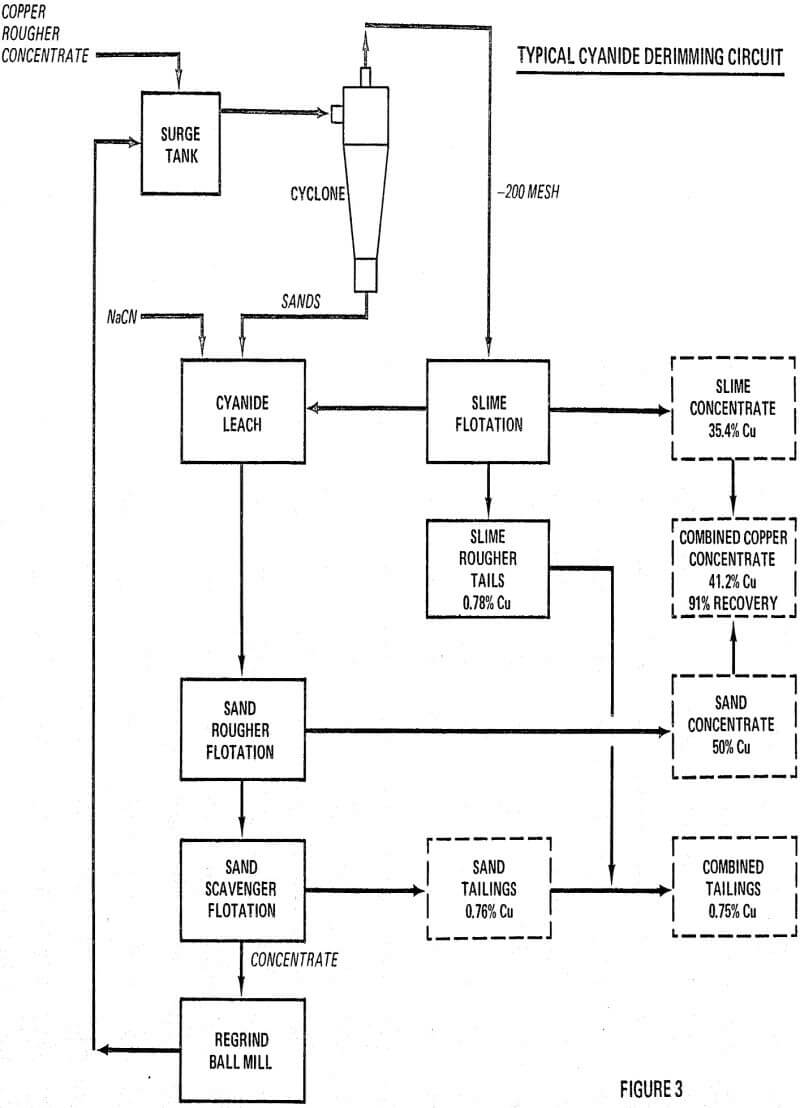
Rougher concentrates from several major copper mills were examined under the microscope. In most cases pyrite rimmed with varying thicknesses of copper sulfides was present. Assay-size analyses of the rougher concentrates were made and theoretical surface areas calculated. On the average, the plus 10 micron fraction, called sands, accounted for 85 percent of the weight, 80 percent of the copper and 10 percent of the surface area. Since the amount of cyanide required to dissolve copper sulfide coatings was directly proportional to the area of copper sulfide exposed, it appeared logical to confine the use of cyanide to the sand fraction. It was recognized that such a sharp sand-slime separation could not be achieved in the usual mill cyclone.
Basic laboratory test procedures consisted of a sand-slime separation in the laboratory cyclone which gave a 70:30 split instead of the theoretical 90:10 ratio.
Present Copper Flotation Practice
Most large mills presently employ excessive regrinding to obtain some degree of selectively. This consumes large amounts of expensive energy in comminution and recirculation pumping of flotation pulp through the cleaner and recleaner circuits. The final concentrates are so fine, they are difficult to dewater on vacuum filters to less than 15% moisture. This increases transportation costs or incurs costs of drying. Despite this intense, expensive processing, final concentrates rarely exceed a grade of 30% Cu.
Theoretically, copper concentrate grade is determined by only one factor—its mineralogical content. A high grade concentrate should contain essentially no free pyrite.
All highly reactive, small chalcopyrite particles must be removed first, usually by cycloning the bulk rougher concentrate. This is the most critical step in the process and hence must be carefully controlled to make the cleanest sand-slime separation at the optimum mesh size selected.
Cyanide Leaching
Only the sand fraction is given a cyanide leach for the purpose of derimming all of the coarse pyrite particles with the thinnest rims. Cyanide consumption will be determined by the amount of chalcocite and other copper minerals dissolved. Experimentation will determine the minimum amount of cyanide necessary to attain the grade improvement desired. It is hence apparent that the cyanide dosage (in terms of pounds used per ton of bulk rougher concentrate) can be used as the method for controlling the final grade of copper concentrate at any desired level. Cyanide consumption will usually be 2 to 6 pounds NaCN per ton of bulk concentrate.
a. A higher grade copper concentrate is produced and zinc and cadmium fuming is reduced at the copper smelter.
b. The zinc can be recovered as a saleable zinc concentrate to produce additional revenues.
Sand Flotation
In the cyanide leach, all of the flotation reagents are usually deactivated, hence additional reagents are needed in the sand flotation circuit. It has been demonstrated that molybdenite can be floated first with the addition of frother and fuel oil. All of the copper minerals can next be floated together using one or more of the standard copper promoters (xanthates-Aerofloats).
Finally, if there is a need for a clean pyrite flotation concentrate, one can be obtained easily from the zinc flotation tailings.
The cyanide leach liquor will contain the copper from the dissolved chalcocite as a non-toxic copper cyanide complex. Tests indicate that usually 2 to 3 percent of the total copper will be dissolved which is small enough for discard.
Indicated Advantages of Cyanide Derimming (Cadwell Process)
Yields high grade copper concentrates (35-55% Cu) which permit significant cost savings in smelting furnace and SO2 recovery operations. Grade can be controlled at any desired level. Reduces sulfur converted to sulfur dioxide and sulfuric acid by more than 50%. Reduces the tonnage of smelter slag to be handled and stored, with attendant reduction of copper losses in the slag. Increases copper recovery in flotation by 2 to 3%.