Table of Contents
- Fluorspar
- Potential Byproducts
- Flotation
- Experimental Materials and Equipment
- Flotation Columns
- Bubble Generator
- Procedure
- Crushing and Grinding
- Fluorite Flotation Circuit
- Mica Flotation Circuit
- Silicate (Beryl) Flotation Circuit
- Zeta Potential Studies Procedures
- Discussion of Results
- Fluorite Flotation
- Process Economics of Fluorite Circuit
- Beryl Flotation
- Column flotation processes designs and practices
- Column Flotation Parameter Testing
We investigated column flotation for recovery of a high-grade fluorite (CaF2) concentrate and byproduct concentrates from the Fish Creek fluorite deposit in Eureka County, NV. The recovery scheme consisted of (1) grinding the ore to minus 48 mesh, (2) fluorite rougher and cleaner flotation, (3) desliming the rougher fluorite flotation tailings at 20 µm, (4) mica flotation, and (5) silicate rougher flotation. Acid-grade fluorspar, mica, free silica (SiO2) sand, and low-grade beryl (Be3Al2Si6O18) concentrate were produced in a 100 lb/h continuous column flotation unit (CCFU). The highest results achieved were 96.6-pct CaF2 recovery in a first cleaner concentrate containing 99.7 pct CaF2. Beryl recovery was as high as 92 pct at a grade of 5.3 pct BeO. The silica sand, which assayed 98 pct SiO2, was recovered as the rougher beryl flotation tailings. Collector reagent consumption was reduced 45 pct for fluorite and 58 pct for silicate flotation in comparison with bench-scale conventional flotation. All products were significantly improved in both grade and recovery over conventional flotation results, and the recovery scheme was simplified by removal of several stages of flotation in the fluorite and silicate flotation steps.
Fluorspar
Fluorspar, the commercial name for the mineral fluorite, defines material that contains a marketable fluorite concentration; or an aggregate of rock (or ore) with the potential to be upgraded to a fluorite content sufficient to allow industrial application. Fluorspar is marketed in three grades, which have different physical specifications and applications:
Metallurgical grade.-Also known as “metspar” or “lump spar,” is used as a flux (slag forming material) in the steel industry. The required effective fluorite content of 60 to 70 pct is calculated by subtracting 2.5 times the percentage of silica from the percentage of fluorite content. Silica content is limited to 5 to 6 pct, sulfides must be less than 0.50 pct, and lead content below 0.25 pct. The silica limit is relaxed to 15 pct SiO2 outside the United States.
Ceramic grade.-Also known as “glass” and “enamel” grade, must contain a minimum of 95 pct CaF2, with a maximum of 2.5 pct SiO2. This material must be fine grained for use in opaque and flint glass, in clay bricks, and in vitreous enamel coatings for metal articles.
Acid grade.-This is used in the manufacture of hydrofluoric acid and should contain a minimum of 97 pct CaF2 and no more than 1.1 pct SiO2, 1.25 pct CaCO3, and 0.03 pct S. Acid-grade fluorite receives the highest price. Uses include the manufacture of Teflon fluorocarbon polymer coatings, fluorine chemicals, synthetic cryolite, refrigerants, and insecticides.
The United States has maintained a high-import reliance (86-90 pct based on apparent consumption) of fluorite for use in the steel, aluminum, uranium, water fluoridation, and chemical industries as a fluorine source. Fluorspar consumption in 1987 was 709,500 st; with a reported acid-grade consumption of 451,000 st, and 147,000 st of metspar. Owing to its strong electronegative chemical characteristics, no substitute has been found for most applications of fluorine, making fluorspar a strategic material.
Fluorine consumption was expected to experience a sharp drop due to an international agreement to freeze and later reduce consumption of chlorinated fluorocarbons (CFC’s) after a hole in the atmospheric ozone was observed. Despite restrictions on CFC’s and the agreement, fluorine consumption increased 12.5 pct to 797,800 st in 1988, including a 30-pct increase in fluorite imports. New substitutes for CFC’s being developed will require more fluorine per molecule and may stabilize or increase consumption.

At projected consumption rates, currently exploited fluorspar deposits of the world are expected to be depleted by the end of the century. As many domestic and foreign deposits of fluorite are exhausted, alternates such as marginal deposits, secondary sources, or multicomponent ores will have to be developed. One such marginal deposit is the Fish Creek fluorite deposit of Eureka County, NV, which contains ore reserves estimated from 2.5 to 5 billion st of ore at a grade in excess of 10 pct CaF2. This represents the United States largest known fluorite deposit.
Potential Byproducts
Beryl
The Fish Creek ore also contains potential byproduct minerals of beryl, mica, and silica sand. Pure beryl mineral contains 5 pct Be or 14 pct BeO. Beryllium is used as an alloy and metal in aerospace and defense uses requiring lightweight, high strength, and high-thermal conductivity in structural and electronic equipment applications. Beryl and beryllium oxide are also used as moderators for nuclear radiation. There are no substitutes that can match beryllium’s performance in these products. The 318 st of beryllium consumed in 1987 were valued near $145 million. Use of beryllium in high-speed circuits and electronic components in cars is rapidly increasing. The National Defense Stockpile is increasing its supply by 60,000 lb.
Most exploration drill cores taken from the Fish Creek deposit were not analyzed for beryllium content, but all samples used in this study assayed between 0.1 and 0.2 pct BeO. If consistent throughout the deposit, this would greatly augment present world beryllium reserves.
Mica
Practical uses for mica (muscovite) are determined by particle size, 100- to 200-mesh scrap, and flake mica is used primarily in building and construction applications such as asphalt products, texture paints, ceiling tiles, concrete block filler, and wall joint cement. Finer sized mica (160-to 325-mesh) is used for paints, and as an additive for plastics to increase strength, heat resistance, or dielectric properties.
Silica Sand
Silica has a wide variety of applications, which grow in proportion to its purity. Abrasives, casting sands for foundries, furnace linings, filter media, glass, ceramics and specialty chemicals all have markets. Prices are low for raw sand, and the marketability of sand is greatly dependent on transportation costs. The most detrimental contaminant is iron, limited to 0.025 and 0.15 pct Fe2O3, respectively, for white and amber glass manufacture.
Flotation
Development of domestic low-grade fluorite resources has been retarded by reliance upon imports from foreign sources, and by inadequate technology for concentrating its low-grade ores. A method for recovering fluorite and byproducts from the Fish Creek ore was developed by the U.S. Bureau of Mines based on previous Bureau research on similar ores using bench-scale froth (conventional) flotation; however, the ore responded poorly to continuous operation. Column flotation has been shown to provide superior results when used in many applications currently limited to conventional flotation processes. An increase in concentrate grade, product recovery, or decrease in the number of flotation stages required to achieve product grade, or a combination of these effects has been documented on several ores examined at a Bureau facility. A study of parameter effects with fluorite ore was carried out and is summarized in appendix B. The advantages of column flotation include improved energy efficiency, utilization of natural settling and separation, reduced turbulence in the flotation cell, and closer control of critical flotation parameters such as bubble size, cell air content, airflow, retention time, froth depth, and froth cleaning. A reduction in reagent consumption using column flotation in comparison with conventional flotation is common. Because of the enhanced results produced by column flotation with other ores, fluorite column flotation tests were performed on a Fish Creek ore sample.
Experimental Materials and Equipment
A 10,000-lb sample was obtained from the Fish Creek fluorspar deposit near Eureka County, NV, which also contains beryllium and muscovite byproduct values. The sample was crushed and screened through ¼ in. thoroughly blended, after which representative portions were submitted for chemical and petrographic analysis. The
chemical analyses are shown in table 1.
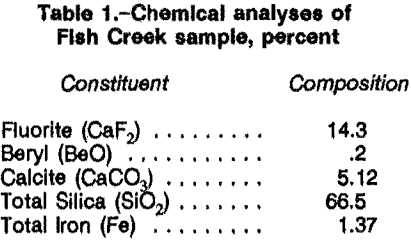
Petrographic analysis of the fluorite beryllium sample indicated that it contained major amounts of quartz (α-SiO2), fluorite, and muscovite (light-colored mica [KAl3Si3O10(OH)2]), Minor amounts of hematite (Fe2O3), limonite (hydrous iron oxides), potassium-feldspar (KAlSi3O8), beryl, calcite (CaCO3), and chlorite (hydrous silicates of aluminum, ferrous iron, and magnesium) were observed. No reliable chemical method was available for determining muscovite concentration, therefore, petrographic analysis was used to estimate mica content. Petrographic estimates for quartz and mica were 28 and 30 pct, respectively. Liberation size for all minerals was 48 mesh.
Flotation Columns
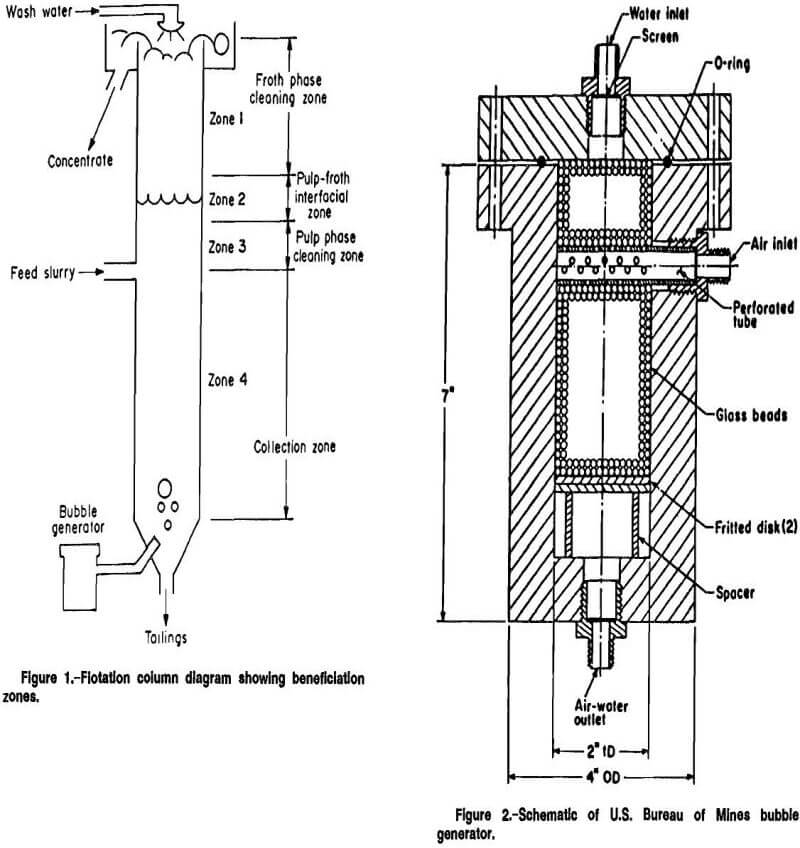
Flotation was accomplished in four 2.5-in diam columns. The rougher fluorite flotation column was 18 ft high; the other three columns (cleaner fluorite, and mica and beryl rougher flotation columns) were 12 ft high. Each column was composed of flanged sections with 1.125-in-diam ports located at 1-ft intervals along its length. Solenoid-actuated sample valves with stainless steel spring loaded plungers were designed and constructed at a Bureau facility and fitted into the ports. These valves permitted rapid, consistent extraction of a representative pulp or froth sample. A concentric cylindrical overflow weir was mounted on the top section of each column. A conical bottom with a 0.5-in-diam port was attached to the bottom section of each column. Figure 1 presents a diagram of a flotation column.
Bubble Generator
The Bureau’s bubble generation system was used to aerate the flotation column. The bubble generator was a 6-in-tall by 2-in-diam clear cylindrical Plexiglas acrylic plastic chamber with 1-in thick walls. This is illustrated in figure 2. The generator had a removable Plexiglas acrylic plastic top attached to the generator body with an O-ring seal. A spacer supported a fritted glass disk in the chamber to prevent short circuiting of air. The remaining chamber volume was Med with 0.04-in-diam glass beads to improve air-water contact. Teflon fluorocarbon polymer chips or quartz gravel, coarsely sized at plus 6 mesh (plus 0.132 in) were substituted for the glass beads with satisfactory results.
A section of 28-mesh screen was placed over each orifice to prevent loss of glass beads. House air was regulated to 60 psig and introduced through the side port of the bubble generator. Water, pressurized to 60 psig by a turbine-blade pump, was introduced through the top bubble generator orifice. Air and water were mixed in the contact chamber; the pressurized mixture exited the chamber through the bottom port and was injected into the column through an aluminum or Teflon fluorocarbon polymer tip with a 0.04-in-diam orifice. The bubbles were
still-photographed inside the column alongside a microgrid and measured after development. The generator design allowed for bubble size control from less than 0.004- to more than 0.12-in average bubble diameter (0.1- to 3-mm) by adjusting air and waterflow rates and by adding Dowfroth 400 frother. Previously published work on this parameter investigation showed coarse bubbles (3- to 5-mm diam) produced the best results on the Fish Creek fluorite ore, and were therefore used for all testing.
An airflow rate of 4,500 cm³/min and a waterflow rate of 800 mL/min were used to generate the 3- to 5-mm-diam bubbles for this testwork.
Procedure
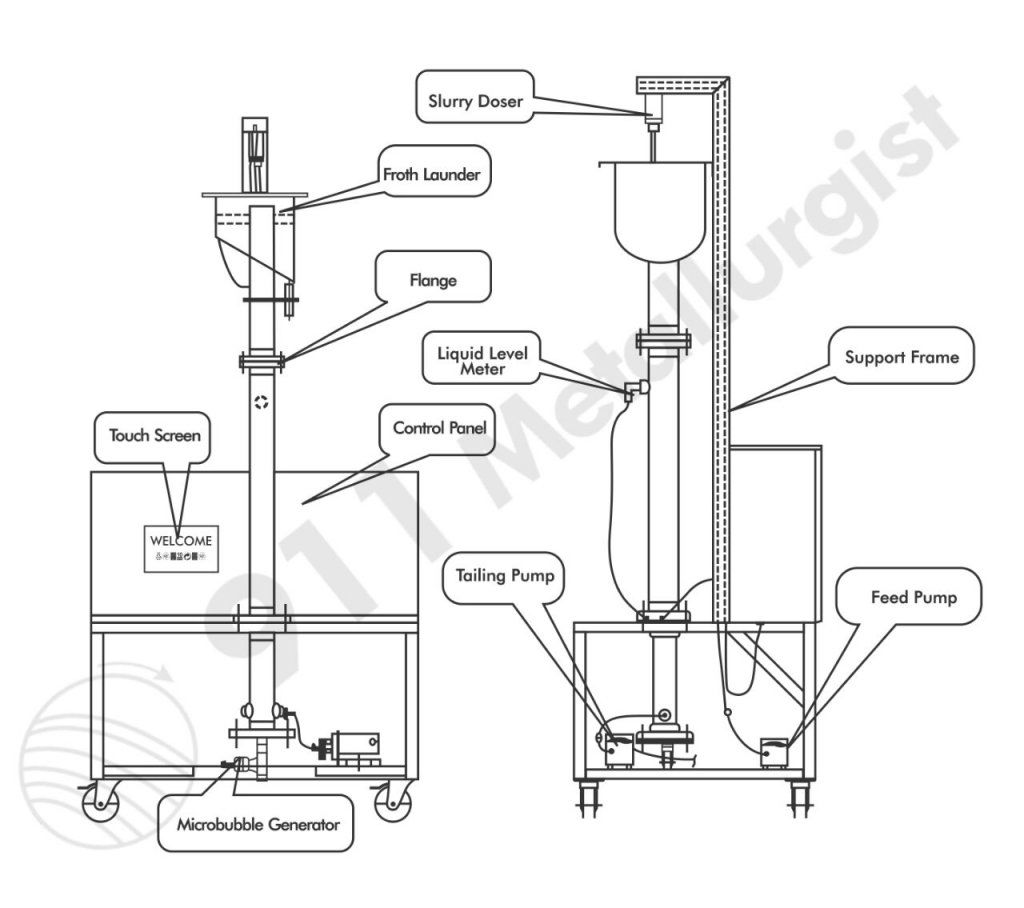
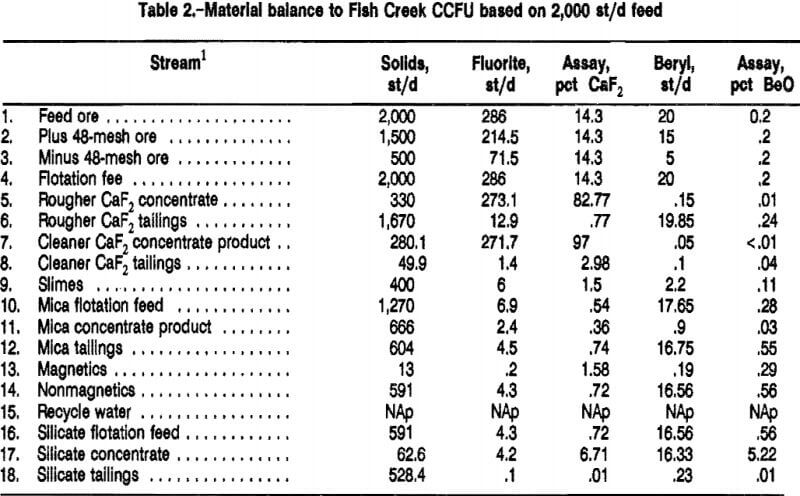
The finalized CCFU flowsheet is given in figures 3 and 4. Table 2 includes the material balance for a 2,000-st/d plant used for the economic evaluation appearing in appendix C. The data are derived from pilot plant results obtained under preferred, sustained operation conditions.
Crushing and Grinding
In the CCFU run-of-mine (as-received) ore, initially crushed to minus ¼ in, was preclassified at a rate of 100 lb/h on a 48-mesh vibrating screen. The plus 48-mesh material was fed directly to the 24- by 36-in rod mill and ground at 50 pct solids using a standard rod charge. The minus 48-mesh material was added to the rod-mill discharge. The undersized and ground oversized material were slurried at 25 pct solids. The grind size distribution of the flotation feed solids is given in table 3.
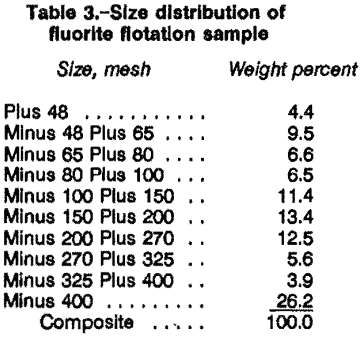
Fluorite Flotation Circuit
Preferred conditions for conventional flotation of the Fish Creek ore determined previously using bench-scale recovery scheme development and mineral parameter testwork, were as follows: 3 lb/st sodium carbonate (Na2CO3), 3 lb/st N-sodium silicate (Na2SiO3), 2 lb/st oleic acid (C18H34O2); with a 15 min conditioning time for each reagent and a flotation pH of 9. When these conditions were directly applied to initial continuous column flotation tests, they were found to be excessive and could be reduced to conditioning with 2.9 lb/st Na2CO3 for 3 min, followed by 2 lb/st Na2SiO3 for 3 min. Oleic acid (dissolved in ethanol) was then added at 1.14 lb/st and conditioned for 3 min, while the pH was maintained near the natural ore pH of 9 with the addition of Na2CO3 and hydrocloric acid (HCl) as needed.
A cascading pilot plant setup was employed to maximize the use of gravity feeding from stage to stage and minimize mechanical difficulties in pumping between process stages. The conditioned fluorite rougher feed material was pumped at 25 pct solids through an agitated feed surge tank to an entry port located 11 ft from the column base using a peristaltic pump and flexible tubing. Mineralized froth continuously flowed from the top of the fluorite rougher column to the feed port located 7 ft from the base in the fluorite cleaner column. Final fluorite concentrate continually flowed by gravity from the cleaner column to a pan-type filter for recovery. Tails from the cleaner column were pumped out, filtered, and discarded.
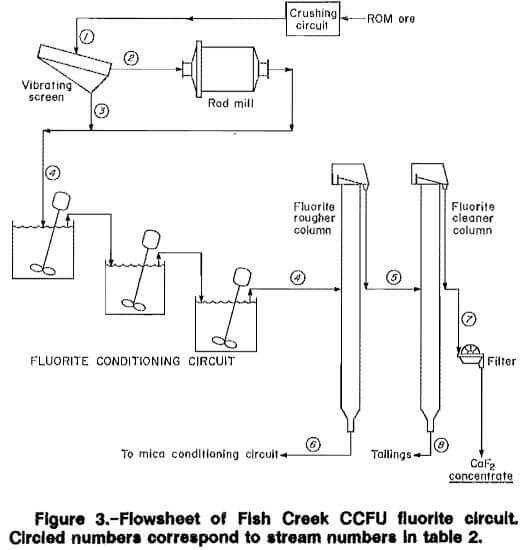
Mica Flotation Circuit
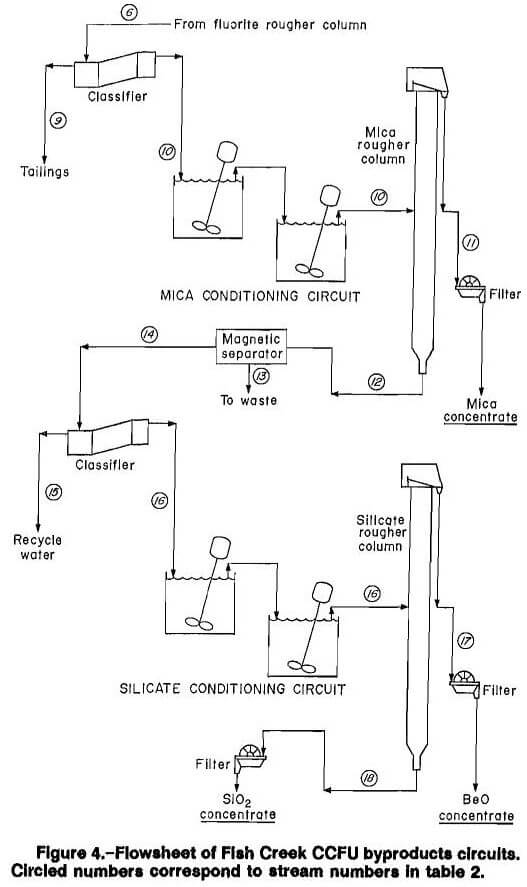
The tailings from the rougher fluorite float were pumped from the base of the column to a spiral classifier for desliming at 20 µm. The minus 20 µm slime material at approximately 7 pct solids was sent to filtration, while the plus 20 µm material was diluted to approximately 20 pct solids and fed to the byproduct recovery circuit (fig. 4), starting with the mica conditioning and flotation circuit.
The preferred method for treatment in the mica circuit consisted of conditioning the deslimed rougher fluorite flotation tailings with 5 lb/st sulfuric acid (H2SO4) for 3 min, followed by 0.75 lb/st primary ether amine acetate (MG-98A) dissolved in ethanol for 3 min, while controlling pH at 2.0 to 2.8 with the addition of sodium hydroxide (NaOH) as needed. This compares with the conventional flotation method in which the feed was conditioned 3 min each with 5 lb/st H2SO4 followed by 0.4 lb/st MG-98A dissolved in ethanol. The conditioned mica flotation feed material was pumped at approximately 20 pct solids through flexible tubing from the conditioner to a port located 7 ft from the column base using a peristaltic pump. Mineralized froth was continuously removed from the top of the mica rougher column and gravity fed to filtration. The tailings from mica flotation were pumped from the base of the column to a magnetic separator for tramp iron removal. After magnetic iron removal, the slurry was sent to a spiral classifier for dewatering ahead of silicate flotation conditioning.
Silicate (Beryl) Flotation Circuit
The preferred method for silicate flotation in the CCFU was to stage condition the spiral classifier product with
4 lb/st hydrofluoric acid (HF) and 0.05 lb/st sodium fluoride (NaF) for 3 min, followed by additions of 0.7 lb/st tallow amine acetate (Armac-T) dissolved in ethanol, and 7.5 lb/st acetic acid (CH3COOH) conditioned for 3 min. The conditioned silicate feed material was pumped at approximately 20 pct solids through flexible tubing from a steady feed tank to a port located 7 ft from the column base using a peristaltic pump. Mineralized froth (silicate concentrate) was continuously removed from the top of the silicate rougher column and gravity fed to filtration. Silicate rougher flotation column tailings consisted almost exclusively of free quartz, a potential glass sand feed material, was pumped directly to filtration.
The original recovery scheme developed by bench-scale conventional flotation testwork involved conditioning with
5 lb/st HF for 3 min, followed by conditioning for 20 s with 1.2 lb/st tallow amine acetate for rougher silicate flotation. The rougher silicate concentrate was then conditioned with 1.2 lb/st sodium hypochlorite (bleach) to remove the amine collector followed by sorption of the organic chemicals onto 12 lb/st activated charcoal, rinsing, and filtration. This was followed by cleaner flotation of the beryl using an emulsion of 0.39 lb/st oleic acid with 0.15 lb/st Triton X-45 emulsifier at a pH of 6.2 using sulfuric acid to depress gangue silicates, and 0.05 lb/st NaF for beryl activation. No beneficiation was observed in the CCFU when this method was employed.
Zeta Potential Studies Procedures
The zeta potential testwork was carried out with pure beryl and quartz samples from Wards Minerals, Rochester, NY. The samples were ground to minus 200 mesh in a ceramic mortar and pestle. In each test, 200 mg of sample was added to 100 mL of pH adjusted, deionized water containing the desired concentration of reagent(s). After 5 min of conditioning, the mixture was transferred into the Riddick cell for zeta potential measurement. All pH adjustments were made with either HCl or NaOH.
Discussion of Results
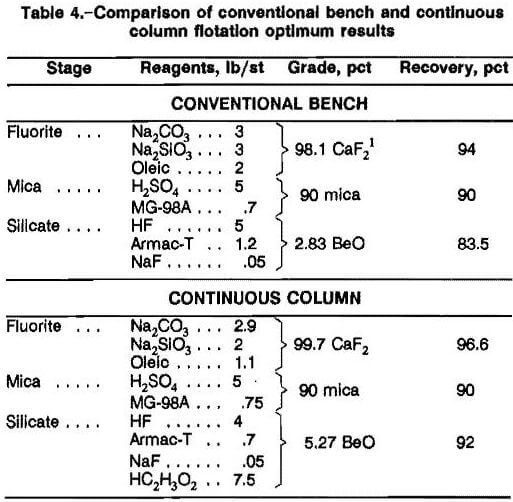
A 100-lb/h CCFU was operated on the bulk Fish Creek sample (figs. 3-4), and removed four products: fluorite, mica, silica sand, and a beryl silicate concentrate. The best results achieved for the CCFU are summarized in table 4 along with a comparison with bench and continuous column flotation results using the same flotation procedure. Reagent additions were unavailable for the conventional silicate flotation circuit. Preferred reagent dosages for the CCFU were 2.9 lb/st Na2CO3, 2.0 lb/st Na2SiO3, and 1.14 lb/st C18H34O2 to the fluorite circuit (pH 9); 5 lb/st H2SO4 and 0.75 lb/st MG-98A in the mica circuit (pH 2.8); 4.0 lb/st HF, 0.7 lb/st tallow amine acetate, 7.5 lb/st CH3COOH, and 0.05 lb/st NaF after magnetically removing the tramp iron from the mica flotation tailings (pH <3).
Fluorite rougher column plug flow retention times based on slurry feed rate and collection zone volume (column volume between feed injection port and air sparger) were approximately 5 min compared with conventional flotation retention times of 15 min. Flow to subsequent processing stages was maintained as necessary to match discharge from the fluorite rougher column.
Using the preferred reagents and conditions in the CCFU, excellent results were achieved. Average cleaner fluorite concentrates of 98.3 pct CaF2 (acid grade) with recoveries of 94.9 pct were achieved. The highest grade and recovery achieved were a 99.7-pct CaF2 concentrate with 96.6 pct recovery. Silica content was less than 0.5 pct; CaCO3, S, and Pb assays were below detection limits (0.1, 1.0, and 0.03 pct, respectively). It was estimated by petrographic analysis that a 90-pct mica concentrate was produced with more than 90 pct recovery. The silicate flotation stage produced two concentrates. The tailings, a silica sand product, always assayed more than 96 to 99 pct SiO2. Iron content around 0.2 pct Fe2O3 prohibits use of this material in glass making, but it would be suitable for use in the manufacture of abrasives, sodium silicate chemicals, and other products. The silicate concentrate produced using the preferred reagent scheme contained 92 pct of the beryl at a grade of 5.3 pct BeO.
The flow scheme for recovery of fluorite and byproducts from the Fish Creek ore was developed in bench-scale testwork reported in Bureau’s report of investigations (RI) 9028. Operation of the CCFU resulted in elimination of (1) two cleaner flotation stages in the fluorite flotation circuit, and (2) a cleaner stage of beryl flotation as well as a reagent removal system requiring sodium hypochlorite treatment, filtration, and activated charcoal treatment. The magnetic iron removal was moved from the rougher silicate concentrate to the mica flotation tailings stream. Column flotation also resulted in higher grades of fluorspar, mica, and beryl products with better recovery.
Fluorite Flotation
Water temperature was found to be an important factor in continuous flotation of fluorite. This effect was unreported in bench studies, probably because of the rapid adjustment to room temperature of small ore charges in contact with flotation machines. Highest recoveries were achieved while maintaining the circuit feed water at 75° F. An 18° F reduction in temperature resulted in a recovery drop of 20 pct or more. The effect seen on the concentrate grade was not as pronounced.
Batch column testing significantly improved the results achieved in bench conventional flotation of the Fish Creek ore. With the columns, adjustment of operating parameters resulted in increased concentrate grades. Manipulation of parameters such as froth depth, wash water addition rate, aeration rate, bubble size, and feed injection location (retention time) had pronounced effects on column performance and metallurgy and were easily incorporated into the CCFU.
Coarse bubbles with moderate air holdups of approximately 12 to 15 pct were chosen as optimal, based on test results prior to CCFU operation. Use of fine bubbles and high-air holdups increased recoveries, but decreased grades as calcite also floated with the fluorite under these conditions.
Column profile samples from a continuous test were analyzed for fluorite, silica, and calcite content (fig. 5). Fluorite was upgraded to the concentrate stream (top of the column), while silica and calcite were rejected to the tailings stream (base of the column). The most efficient separation of fluorite from silica occurred in the froth phase cleaning and pulp-froth interfacial zones (figs. 1 and
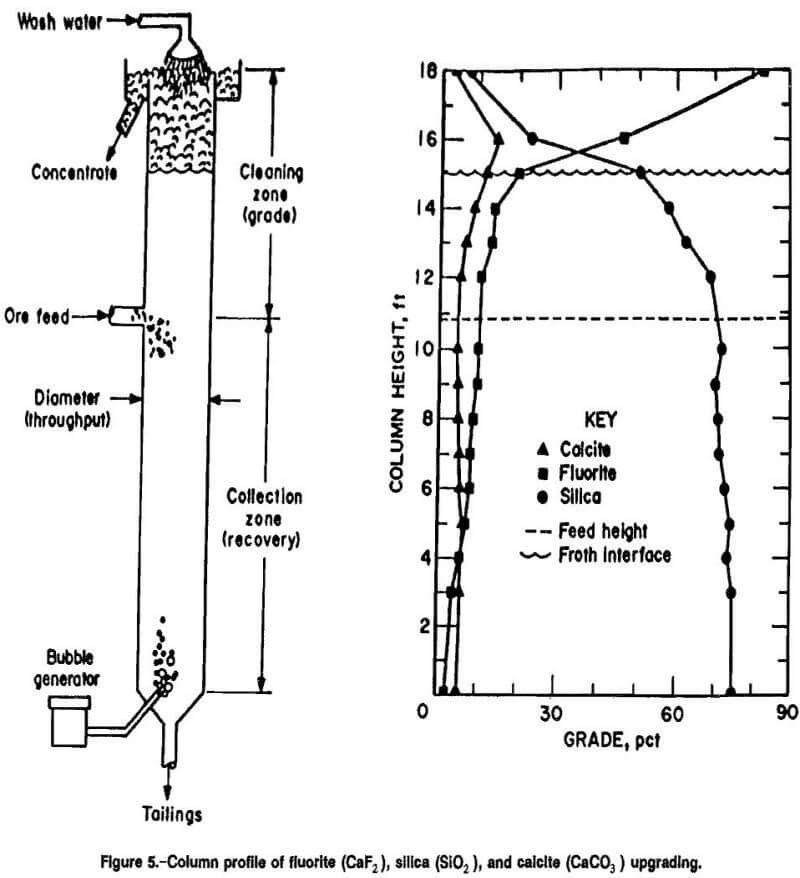
5). This suggests that the specific function of zones 1 and 2 is upgrading, linking them strongly to mineral concentrate grade, while zones 3 and 4 predominantly act as collection zones, linking them specifically to mineral recoveries.
Calcite behavior during column flotation was particularly interesting because the flotation response of calcite mirrored that of the collected fluorite and that of the rejected silica at different points within the column. Calcite concentrated with the fluorite in the lower three column benefieiation zones. Calcite rejection was accomplished in the froth phase cleaning zone. Unlike these results in the CCFU, continuous and bench conventional froth flotation were unable to reject the entrapped calcite.
The fluorite rougher column employed low wash water and shallow froth beds (<2 ft) to achieve good grades with high-fluorite recoveries. The fluorite cleaner column used 6 ft of froth and high wash water addition rates (>100 pct of feed slurry flow rate) and achieved substantial upgrading of the fluorite with rejection of the silica and calcite. Feed was injected high in the fluorite rougher flotation column (to achieve high recovery) and lower in the fluorite cleaner column (where bubble particle attachment was rapid) where grade was the primary objective. With this, 99 pct CaF2 grades were attainable.
Maintenance of high-fluorite recoveries was necessary for improved downstream byproduct grades. Conventional flotation would have required multiple stages of scavenger and cleaner flotation to equal the higher grades achieved using column flotation. Use of Plexiglas acrylic plastic in construction of the columns also allowed close monitoring of froth heights and bubble generator performance. Close control of aeration rate in the mica flotation column prevented production of an uncontrollable froth (which occurred under high aeration conditions with the amine flotation chemistry) and resulted in higher purity mica concentrates because of less gangue entrainment.
Process Economics of Fluorite Circuit
Based on the promising results achieved with column flotation, the Bureau conducted a cost evaluation on column flotation to recover fluorite only from the Fish Creek deposit. The evaluation concluded that a 2,000-st/d column flotation mill could operate with an estimated 12 pct return on investment after taxes primarily due to the higher price commanded by the high-purity fluorite product.
This process evaluation considered the marketability of fluorspar only because markets for mica, beryl, and glass sand were too small to justify processing the entire tailings stream for byproducts. The evaluation was formulated with conservative estimates to assure reliability, and did not take land investment, mineral depletion allowance, or plant owner’s supervision cost into account. A Marshall and Swift (M & S) index of 897.0 was used for the evaluation estimates, performed in the third quarter of 1989. Details of the economic evaluation for column flotation of fluorite are presented in appendix C.
Beryl Flotation
Several problems were encountered in the flotation of beryl in the CCFU. Iron minerals and iron stained quartz were difficult to reject with the cationic (amine) collector system. Even with the use of HF to condition the mineral surfaces, close control of collector addition had to be maintained to produce a concentrate superior to that produced using conventional froth flotation methods.
Even the presence of high levels of iron ions in the water had to be prevented to obtain selectivity in beryl flotation. Iron contamination from mild steel conditioning tanks interfered with beryl flotation selectivity and had to be replaced with stainless-steel tanks. Iron activation of minerals in silicate flotation has been reported by other investigators, as well as the difficulties in beryl flotation with fatty acids and other collectors. No process has reported success in producing a suitable beryl concentrate (BeO content of 10- to 11-pct, where pure beryl has a BeO content of 14 pct) strictly using flotation. All beryllium production has been accomplished with hydrometallurgical processes from bertrandite and high-grade beryl sources.
Beryl concentration from the low-grade ore in the CCFU was also hampered by the greatly decreased flow rates at the end of the process, and the time required to accumulate enough material to begin the unit operation. Separation of iron-stained quartz and iron minerals was difficult due to poor selectivity of the collector (tallow amine acetate).
Incorporation of the initial recovery scheme, including a reagent removal scheme followed by beryl flotation of the silicate concentrate with an oleic acid emulsion, did not improve CCFU beryl grades. In bench flotation, a 5.96-pct BeO concentrate with a recovery of 65.8 pct of the beryl values was achieved; but in CCFU operation the cleaner beryl flotation increased grade from 1.76 pct BeO to only 3.35 pct BeO with poor recovery. The continuous conventional system based on the original scheme was unable to exceed 1 pct BeO due to material control problems and inferior recoveries.
Zeta potential studies were performed on pure quartz and beryl samples to find conditions allowing improved quartz-beryl separation. Effects of HF, NaF, and C18H34O2 emulsion were examined with varying pH and also varying concentrations of HF and C18H34O2 emulsion. Results of this basic research were not completely applicable to beryl flotation of the actual ore because of the use of pure minerals and the absence of iron in the tests.
The surface charges of beryl and quartz were nearly identical without reagents (fig. 6), with the only difference lying in the pH range of 2.0 to 2.5, where beryl still has a
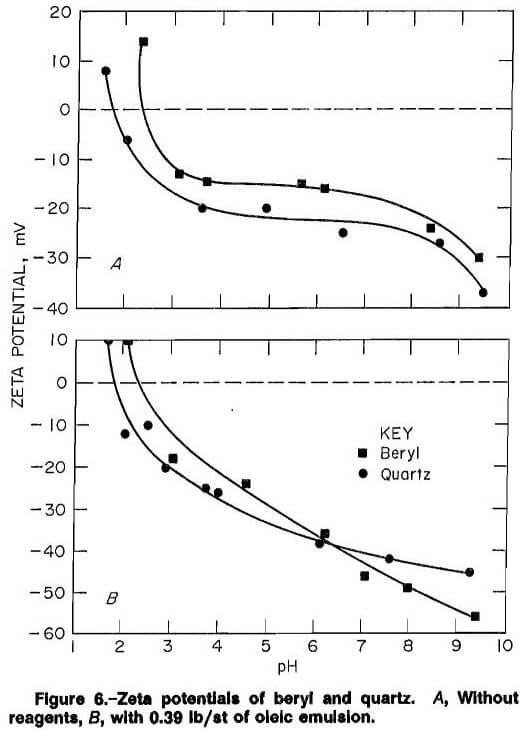
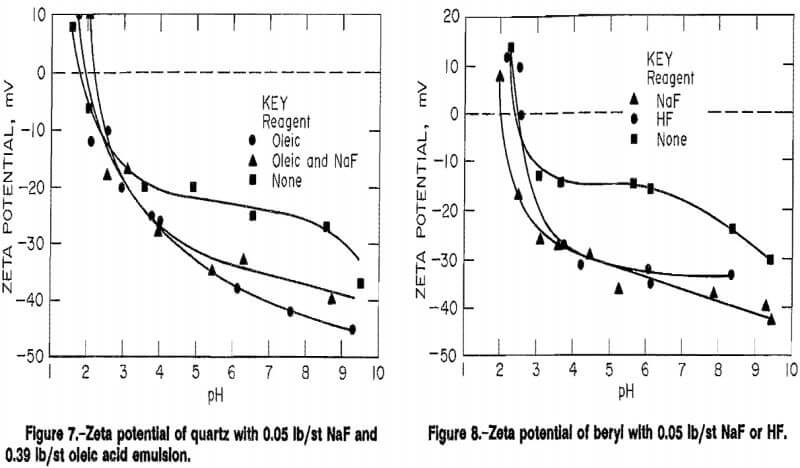
slightly positive surface. In the presence of 0.39 lb/st oleic emulsion (fig. 7), the surfaces remain similar, but the beryl surface charge was more negative above a pH of 4 due to collector adherence to the surfaces. Adherence to the quartz surface was also quite strong showing oleic acid to be nonselective between beryl and quartz, supporting data from the CCFU.
Fluoride additions had the strongest effects on the mineral surfaces. Below a pH of 2.3, quartz showed an extended range of positive surface in the presence of 0.05 lb/st NaF and 0.39 lb/st oleic emulsion (fig. 7). This indicates a resistance to oleic acid attachment to the surface. Use of HF decreases the pH range in which beryl exhibits a positive surface charge (fig. 8). Anionic flotation of beryl would be possible in this region, but acid consumption would be high. The difference between the curves is small and lies in the region of lowest precision for the instrument. Overall, these data all point to a poor applicability of anionic (fatty acid) flotation for separation of beryl and quartz.
Addition of acetic acid to the CCFU silicate flotation circuit aided separation of the beryllium silicate from the silica sand, increasing the rougher concentrate grade from approximately 1.8 pct to 5.0- to 5.5-pct BeO. The increase in selectivity is believed to be due to improved dispersion and decreased amounts of collector attached to particles allowing the column flotation system to reject particles containing iron or silicate middlings. No significant decrease in beryl recoveries was noted, and silicate flotation tailings assays increased by only 0.01 pct BeO or less. Control of froth depth and wash water addition were used to obtain maximum BeO grade and recovery depending on silicate column froth characteristics. This physical process manipulation was not possible in conventional froth flotation.
Increased additions of acetic acid and cleaner flotation stages in the beryl flotation circuit showed minimal improvements, recovering 70.7 pct of the beryl at a grade of 7.36 pct BeO in a second cleaner concentrate with a total addition of 20 lb/st CH3COOH. The amine attached to the iron stained particles was too tenacious for the wash water and acetic acid to overcome.
Column flotation processes designs and practices
Based upon results obtained in operating the continuous column flotation unit with Fish Creek ore, it is concluded:
- An acid-grade fluorite product can be recovered with rougher and a single stage of cleaner flotation using flotation columns with bubble generators developed by the U.S. Bureau of Mines.
- Muscovite and silica sand byproducts of commercial grade can also be recovered by column flotation of the deslimed rougher fluorite tailings.
- Column flotation can control flotation grades and recoveries more closely by providing greater flexibility of operation parameters including: (1) controllable froth depth; (2) addition of wash water to enhance removal of mineral particles only slightly bound by collector to the bubbles in froth phase; (3) minimization of turbulence that may promote bubble particle detachment; and (4) control of retention time by variation in feed injection location and column height.
- Water should be kept at room temperature or higher for optimum fluorite grades and recoveries.
- Acetic acid addition improved selectivity of the amine acetate collector in beryl flotation.
