Table of Contents
- Roast-Leach Process Description
- Experimental Results
- Summary and Conclusions
- Appendix A.- Process Chemistry
- Appendix B Equipment and Procedures
- Horizontal Batch Reduction Roast
- Leaching
- Solvent Extraction and Electrowinning
- Appendix C Product Description and Feed Preparation
- Oxidized Calcine Description
- Bulk Reduced Calcine Description
- Appendix D Oxidation Roast-Material Balance-and-Cost Data
- Material Balance
About 85 percent of the copper produced in the United States is derived from copper sulfide ores by flotation, smelting, and electrolytic refining. Chalcopyrite is the predominant copper sulfide mineral in vein and disseminated porphyry-type deposits. Projected production of chalcopyrite concentrates from copper deposits now being developed and from expanded operations will soon exceed existing smelting facilities. Expansion of current smelters or construction of new facilities for processing concentrates by smelting or other means is probable.
Sulfur dioxide emission problems at existing smelters is a concern to the copper industry, and these problems have been reviewed by several authors. In some U.S. smelters, copper sulfide concentrates are preroasted, eliminating about 50 percent of the sulfur in a 10 to 15 percent SO2 offgas that is mixed with converter gas and used to produce sulfuric acid. The partially roasted calcine, flux, and converter slag are charged to a reverberatory furnace for melting and separation into matte and slag. Preroasting increases the reverberatory furnace smelting capacity and permits, in the case of fluid-bed roasting, the recovery of about 80 to 85 percent of the SO2 gas rich enough for acid making. Those copper smelters that do not preroast the copper sulfide concentrate emit 30 to 40 percent of the sulfur as SO2 in the reverberatory gas. Whether or not the concentrates are preroasted, the reverberatory gases are too dilute for acid making.
Various techniques have been proposed for preroasting copper sulfide concentrate to obtain a rich gas suitable for acid making or reduction to sulfur and a calcine suitable for hydrometallurgical processing. To obviate problems from insoluble ferrites formed in dead roasting and of sulfate disposal in sulfation roasting, a combination oxidation-reduction roasting procedure was developed by the Bureau of Mines. The calcine so produced was amenable to processing by ammonia leaching, solvent extraction, and electrowinning. A few tests and analyses were made to generally track components other than copper, iron, and sulfur as the copper sulfide concentrate was treated in the process. Rhenium was volatilized during the oxidation roast and was collected in the filter bag dust. With some copper concentrates, this product might merit a byproduct treatment prior to recycling the filter bag dust back to the oxidation roaster. Molybdenum, lead, gold, and silver reported to the ammoniacal leach residue, and only a minor amount of zinc was solubilized. A cyanide leach of the residue extracted over 80 percent of the gold and silver, but cyanide consumption was high due to the copper content of the residue. Pioneering flotation tests on the leached residue indicated that at least 50 percent of the copper can be recovered in a product of sufficient grade for recycling to the pelletizer and oxidation roast.
A preliminary cost estimate of the proposed process was prepared based on the conjectured extrapolation of laboratory data to a plant treating 1,000 ton-per-day of 28.8 percent copper concentrate. This estimate is compared with treatment of the concentrate in a conventional smelter.
Roast-Leach Process Description
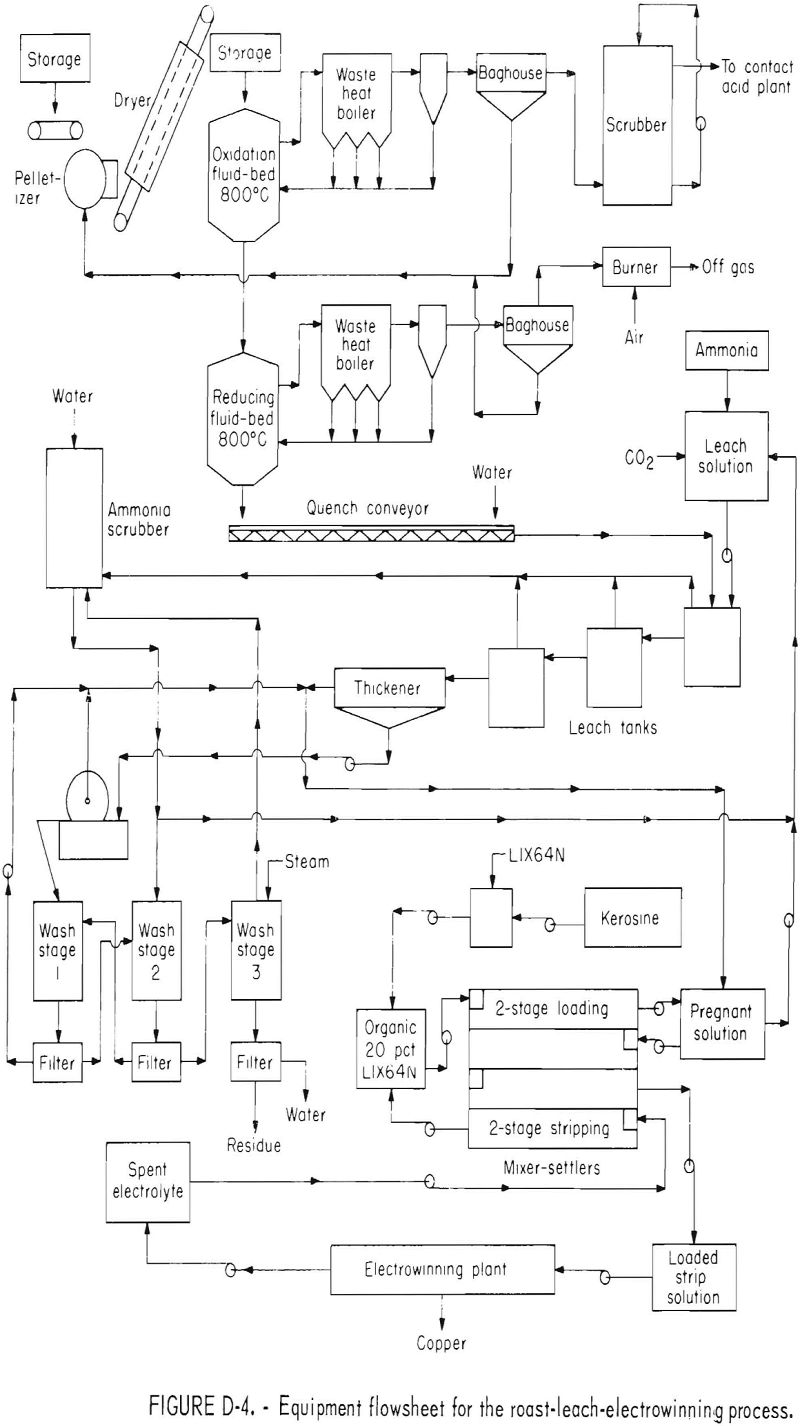
The process, shown in figure 1, starts with a first stage fluid-bed oxidation roast of pelletized chalcopyrite flotation concentrate to remove the sulfide sulfur in an SO2 offgas of sufficient strength for conversion to acid or elemental sulfur. Air, or a mixture of air, oxygen, and recycled SO2 gas would be used as the fluidizing gas. The oxidized calcine, composed mainly of copper ferrites, is fed directly into the second fluid-bed reactor to minimize heat losses. The reducing fluidizing gas in the second fluid-bed reactor converts the copper ferrites into finely divided metallic copper and iron oxides. Offgas from the reduction reactor contains substantial quantities of unused reductants which could be burned as fuel.
The reduced calcine is treated by ammoniacal leaching to solubilize the copper. After filtration and washing, the leach residue is discarded or separately treated for precious metal recovery. Pregnant solution goes to a storage tank, is mixed with raffinate, and subsequently treated by solvent extraction to transfer the copper to the organic. The raffinate from solvent
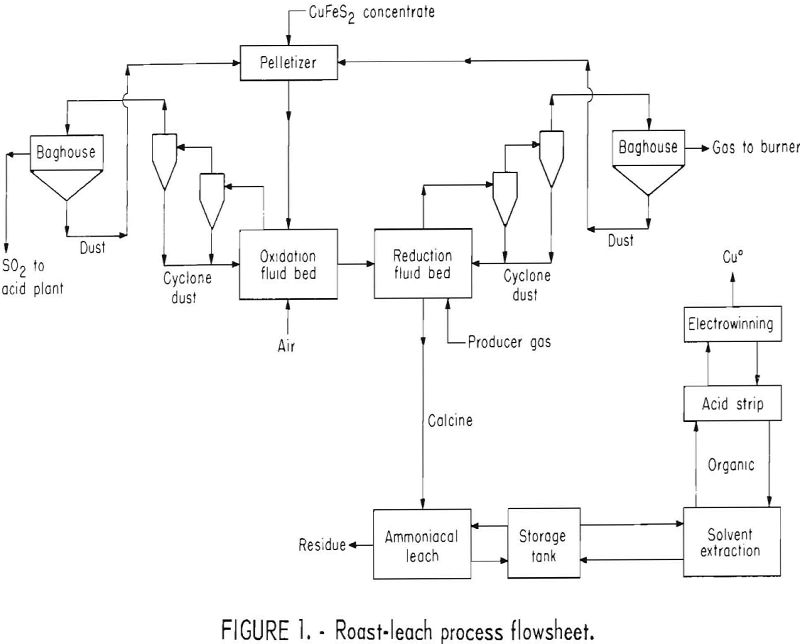
extraction enters the same storage tank and then returns to the leaching circuit. The loaded organic is stripped of its copper by contacting with spent acid electrolyte from copper electrowinning. Loaded strip liquor feeds an electrowinning cell where metallic copper is deposited on the cathode, and acid solution for stripping is regenerated at the anode.
Experimental Results
A copper sulfide concentrate containing, in percent, 28.8 Cu, 28.0 Fe, and 31.8 S, and being generally typical of a concentrate produced by flotation of copper porphyry ores, was used in the investigation. The concentrate was pelletized to minus 20 mesh plus 65 mesh to reduce dust carryover in the fluid-bed reactor, and silica sand, 10 percent by weight, was added to the pellets to abrade coatings that tended to form on the reactor walls.
Oxidation Roast
Pioneering experiments indicated that high SO2 concentration during the oxidation roast could be effective in reducing ferrite formation. A detailed study was made of the effect of SO2-O2 atmospheres on the oxidation of chalcopyrite flotation concentrates. The study showed that retention time, roast temperature, and sulfur dioxide content of the fluidizing gas had interrelated effects on the type of calcine produced. The effects of retention time and temperature were similar in that increases in these variables increased the decomposition of the copper and iron sulfates and favored the formation of copper ferrite. Increases in the concentration of sulfur dioxide in the fluidizing gas had the opposite effect in that ferrite formation was reduced and decomposition of the sulfates was retarded.
The research showed that basically two types of calcines could be produced. The use of a high-sulfur dioxide fluidizing gas in a low temperature roast converted most of the copper to a soluble sulfate. A copper sulfate calcine containing 95 percent water-soluble copper and an offgas containing 85 percent SO2 were produced by roasting chalcopyrite concentrate at 650° to 700° C in a gas initially containing, in percent, 70 SO2 , 21 O2 , and 9 N2. About 50 percent of the sulfur in the feed was evolved in the strong gas by this type roast. The use of lower sulfur dioxide concentrations in the fluidizing gas and higher temperatures resulted in a calcine containing most of the copper in the form of oxide and ferrite. Oxide-ferrite calcines containing less than 5 percent water-soluble copper and 60 to 65 percent acid- soluble copper were made by roasting at 800° C in fluidizing gases containing 0 to 25 percent SO2, 21 percent O2, and the remainder nitrogen. Offgases, containing 12 to 37 percent SO2, were produced, and over 95 percent of the sulfur was removed from the copper concentrate. The stack gases from both the sulfation and dead roasts should be suitable for conversion to elemental sulfur or sulfuric acid.
Treatment of the sulfated copper by hydrometallurgical techniques results in a buildup of dilute sulfuric acid in the leaching circuit. Removal of the acid is generally done by leaching copper oxide ores when available, or by neutralization with limestone. The sulfur in both cases becomes a waste product. Because few copper concentrate processing plants have oxide ores available and because of the loss of the sulfur, emphasis was placed on a process for treating the dead roasted copper ferrite product. By incorporating a reducing roast in the process, the insoluble ferrite would be converted to ammonia soluble copper metal.
Continuous fluid-bed oxidation roasting of the pelletized chalcopyrite concentrate at the optimum conditions of 800° C and 1.5 hours retention time produced a calcine which contained, in percent, 28.7 Cu, 25.9 Fe, and 1.7 S for use in reduction roast studies. The cyclone product was recycled resulting in the collection separately of reactor overflow and filter bag products. Total weight reduction was 12 percent of the combined copper concentrate and silica sand feed weight with 96 percent of product weights being collected in the reactor overflow. Only the overflow calcine was used for the reduction studies. Size analysis showed the calcine was 64 percent plus 100 mesh and only 20 percent was minus 200 mesh.
Reduction Roast
Three reduction roast procedures were used during the course of the investigations, batch horizontal boat, continuous fluid bed, and batch fluid bed. Initially, batch boat roasts were run at temperatures from 600° to 800° C, and for retention times of 1 to 4 hours to evaluate temperature and time variables in reduction of copper ferrite to metallic copper. Degree of reduction or metallization was determined by a standard ammoniacal carbonate leach.
A sweep gas containing, in percent, 3.9 H2, 13.8 CO, 12.3 CO2, and 70.0 N2 was used as the reductant. The gas composition is similar to the offgas from continuous fluid-bed roasts using producer gas as the reductant and fluidizer. Table 1 is a summary of the results.
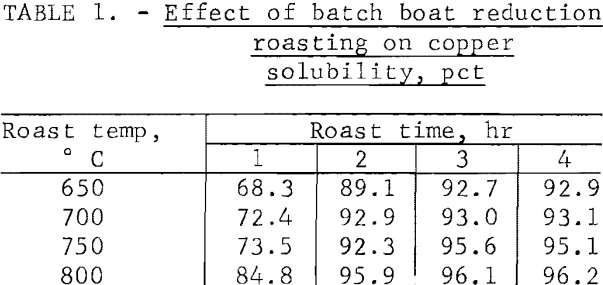
Optimum conditions for roasting the concentrate were found to be between 750° to 800° C for 3 hours resulting in copper extractions exceeding 95 percent. Because diffusion of the reducing gas was somewhat impeded by the ½-inch thickness of the calcine in the ceramic boat, it was believed that metallization would be even higher when reducing the calcine in a fluid-bed where gas-solid contact would be optimized.
Batch and continuous fluid-bed reduction tests were conducted using a simulated producer gas assaying, in percent, 12.5 H2, 20.5 CO, 7.5 CO2, 3.0 CH4, and 56.5 N2 as the fluidizer and reductant. The cyclone dust was recycled. Results are shown in table 2. Because of short circuiting, only 88 percent copper solubility was obtained in the continuous tests. Aerating during leaching increased the copper extraction to 90 percent, but changing the operating condition of the continuous fluid-bed had no significant effect. The short circuiting might be prevented in larger reactors by adding baffles, but this was not feasible in the small reactor. Batch fluid-bed tests showed, when short circuiting was kept at a minimum, that good reduction of the oxidized calcine could be obtained and that batch boat reduction tests could be used to indicate the optimum operating conditions.
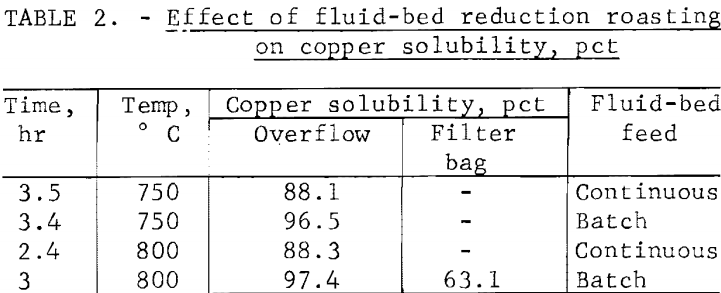
To provide samples for leaching and solvent extraction research, several batch fluid-bed reduction tests were run at 800° C for 3 hours using the simulated offgas composition as the reductant and fluidizer. The cyclone fines were continuously recycled to the reactor bed, and only an overflow calcine and filter bag products were collected. An ammoniacal leach extracted 97.4 percent of the copper from the overflow calcine, but only 63.1 percent from the filter bag dust. Thus, the filter bag product would need to be recycled to the pelletizer in a continuous process. Overall weight loss in the batch fluid-bed tests averaged 15.2 percent compared to 11 to 15 percent in the continuous fluid-bed tests, and the filter bag product weighed 7 percent of the feed weight in the batch tests compared to 5 to 10 percent in the continuous tests. The overflow calcine assayed, in percent, 32.7 Cu, 29.4 Fe, and 0.4 S, and was used without further treatment in leaching studies.
Leaching
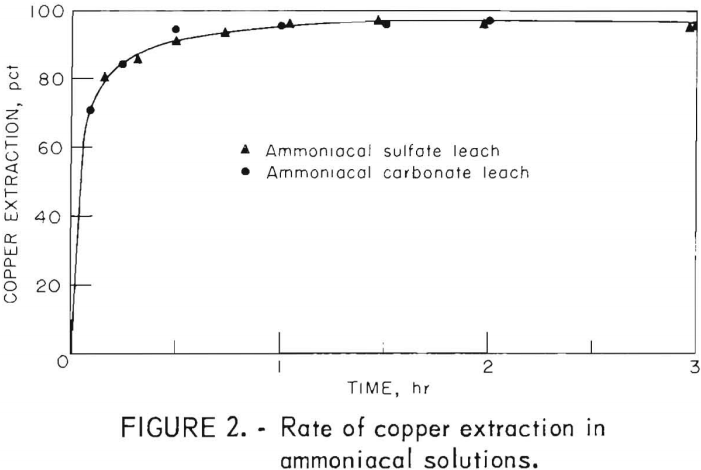
Agitation leaching of metallic copper in ammoniacal carbonate solutions has been studied by several investigators. Their findings corroborated by our work on reduced calcine was that the maximum rate of copper extraction is obtained when (1) the initial copper concentration in the leach solution is approximately half the final copper concentration of the pregnant liquor; (2) a slight excess of total ammonia is used over the required stoichiometric amount to complex all the copper in the pregnant liquor; and (3) the initial amount of free ammonia in the leach solution is equal to or greater than the ammonia needed as carbonate to complex the copper leached. To prevent excessive ammonia losses via evaporation, leach solution should not contain more than 15 grams per liter of free ammonia. Under these guidelines, batch tests were run at room temperature and pressure using both carbonate and sulfate ammoniacal solutions. No difference, shown in figure 2, was noted in either the rate or amount of copper extracted. The sulfate system is superior because (1) some sulfate is leached from the reduced calcine and serves to make up for losses; (2) sulfate losses from evaporation would be less than carbonate; and (3) ammonium sulfate is lower in cost and easier to handle than the carbonate.
The batch sulfate leach solution contained, in grams per liter, 15 Cu, 35 NH3, and 45 SO4, and as seen in figure 2 the maximum copper extraction of 97.4 percent was achieved in 1.5 hours. A continuous three-stage concurrent
agitation leach gave similar results and provided pregnant liquor for solvent extraction tests. The pregnant liquor assayed, in grams per liter, 30 Cu, 34.7 NH3, and 45.0 SO4. The residue weight was 65.5 percent of the reduced calcine feed weight and assayed 1.25 percent copper.
The pregnant liquor was mixed with synthetic raffinate assaying, in grams per liter, 35 NH3, and 45 SO4, to give a liquor assaying, in grams per liter, 15 Cu, 35 NH3, and 45 SO4. This liquor was split with half going to the solvent extraction circuit and half to the reduced calcine leaching step.
Solvent Extraction
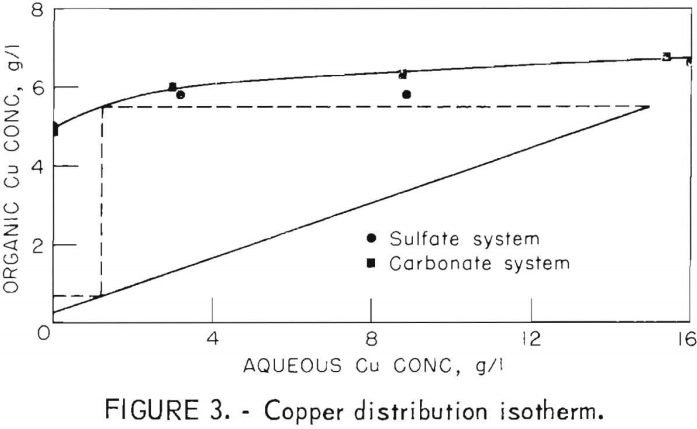
Solvent extraction behavior of copper contained in ammonium carbonate and ammonium sulfate was compared in shaker tests using a 20-percent solution of LIX 64N in kerosine as the extractant. The results shown in figure 3, indicate that copper loading in the organic was slightly higher from the carbonate solution, but in general, the systems did not differ significantly. Saturation loading of the organic in contact with ammonium sulfate containing 15 grams Cu per liter was 6.6 grams Cu per liter.
The continuous solvent extraction circuit using two stages each of extraction and stripping is diagrammed in figure 4. Extraction of a 15-gram Cu-per-liter solution at a 3:1 organic-to-aqueous ratio yielded a raffinate containing less than 0.001 gram Cu per liter and a loaded organic containing 5.5 grams Cu per liter. A McCabe-Thiele diagram imposed on figure 3 indicates only two theoretical extraction stages would be required.
The two-stage continuous-stripping circuit was operated at a 1:1 organic-to-aqueous ratio. The strip solution was spent electrolyte from the electrolysis circuit and contained, in grams per liter, 25 Cu and 100 H2SO4. Five grams of copper per liter were stripped from the organic which increased the aqueous to 30 grams copper per liter. Transfer of ammonia resulted in a buildup of ammonia in the acid strip to 3.1 grams per liter in 20 hours of operation. In commercial operations using similar solvent extraction techniques, the ammonia content levels out at about 30 grams per liter in the electrolyte when an ammoniacal carbonate leach system is used. Similar levels of ammonia should be expected in the sulfate leach system with prolonged operation but should not prove to be a problem in the electrowinning circuit.
Electrowinning
The electrowinning cell was operated in a closed circuit with the solvent extraction stripping circuit at an aqueous feed rate such that the cell volume

was displaced every 35 minutes. The aqueous feed contained 30 grams Cu per liter from which 5 grams were removed in the electrolytic cell. The cell was of special design which was capable of higher current densities than the typical electrolytic cell. A current density of 30 amperes per square foot of cathode produced a solid, dense, and adherent copper plate. Current efficiency averaged 93.7 percent, and the power consumption was 1.02 kW-hr per pound of copper plated. Impurity content of the product was, in percent, 0.003 Pb, 0.001 Fe, 0.001 Si, and 0,001 Mg.
Summary and Conclusions
The double roast-leach process as described in this report appears capable of processing copper sulfide concentrate to make electrolytic copper and an offgas rich in SO2. Preliminary estimates indicate costs would be competitive with conventional smelting only when consideration was given to byproduct recovery. The optimum conditions for the first-stage oxidation roast of pelletized chalcopyrite concentrate are a retention time of 1.5 hours at 800° C using air as the fluidizing gas. This results in 95 to 98 percent sulfur elimination in an offgas assaying 12 to 14 percent SO2, which is of sufficient strength for conversion to sulfuric acid or direct reduction to elemental sulfur. The oxidized calcine composed mostly of cupric ferrite can be reduced to metallic copper in the fluid-bed using producer gas as the reducer and fluidizer. The optimum conditions of reduction are a retention time of 3 hours at 800° C resulting in a calcine from which 97.4 percent of the copper was extracted by a 1.5-hour ammoniacal sulfate leach. Solvent extraction of the copper using LIX 64 N and electrowinning from the sulfuric acid strip solution yielded cathode copper of 99.99 percent purity. Although solvent extraction and electrolytic operations can be scaled to plant size from laboratory data, larger scale test work would be needed to verify laboratory results obtained in engineering design data for the fluid-bed roasting operations.
Appendix A.- Process Chemistry
Roasting
Sulfation roasting of chalcopyrite in air to convert all the copper to soluble sulfate was investigated in a pilot plant operated by Bagdad Copper Corp. in Arizona in the mid-1950’s. Five tons per day of chalcopyrite concentrate and up to 750 pounds per day of cement copper from dump leaching were processed by sulfation roasting, leaching of the water soluble copper sulfate, and electrodeposition of copper from the leach liquor. In the sulfation roast, half of the sulfur was converted to sulfur dioxide in an offgas suitable for conversion to sulfuric acid. The sulfur accompanying the copper sulfate in the leach solution was converted to sulfuric acid during electrowinning. This was mainly excess acid that would require neutralization with limestone before the spent electrolyte could be recycled to the calcine leaching step. Although not practiced at Bagdad, a part of the spent electrolyte presumably could be injected into the roaster as a coolant and the contained sulfuric acid decomposed to yield sulfur dioxide. However, more spent electrolyte would be produced than could be used in this manner.
Another method of consuming the acid in the spent electrolyte is by leaching oxide ore or mine waste dumps. This procedure was piloted by Hecla-ElPaso at their Lakeshore property in 1972-73, and plans are being made to expand to a full-size plant. However, this procedure is only applicable to those properties where oxide reserves are substantial.
Roasting of chalcopyrite concentrate to the oxide appears to bypass most of the problems associated with sulfate roasting. Nearly all the sulfur is removed as sulfur dioxide with very little sulfate remaining in the calcine to create acid buildup in a leach circuit. The roaster gas could be processed to produce sulfuric acid or elemental sulfur. However, when iron-bearing minerals are present in the concentrate, an oxidizing roast produces copper ferrites that are difficult to dissolve in acid solution. Copper and iron sulfates formed by the oxidation roasting of chalcopyrite decompose to copper oxide and iron oxide which react to form copper ferrite according to the following simplified reactions:
CuSO4 ↔ CuO + SO2 + ½ O2 ……………………………………………………………………….(A-1)
2FeSO4 ↔ Fe2O3 + 2SO2 + ½ O2 ………………………………………………………………..(A-2)
and CuO + Fe2O3 ↔ CuO·Fe2O3…………………………………………………………………..(A-3)
The conversion of up to 50 percent of the copper into ferrites that are insoluble in spent electrolyte is an important reason why oxidation roasting has not been used commercially for treatment of copper sulfide concentrates. The effect of sulfur dioxide in the oxidizing gas on both dead and sulfation roasting of chalcopyrite can be seen in the following reactions taking place in the fluid-bed reactor:
2CuSO4 ↔ CuO·CuSO4 + SO2 + ½ O2…………………………………………………….(A-4)
and CuO·CuSO4 ↔ 2CuO + SO2 + ½ O2………………………………………………….(A-5)
Copper sulfate (CuSO4), copper oxysulfate (CuO CuSO4), and copper oxide (CuO) are products of the oxidation roasting of chalcopyrite. With high SO2 concentrations, reactions A-4 and A-5 are driven to the left, resulting in more water-soluble copper sulfate and less water-insoluble copper oxide and copper oxysulfate. High concentrations of sulfur dioxide tend to override the effect of high temperature, thus reducing ferrite formation and leaving most of the copper in the form of acid and water-soluble sulfates.
When dead roasting the copper concentrate at 800° C, the type of copper ferrite appearing in the calcine was found to be dependent on the amount of excess oxygen in the fluidizing gas. When the oxygen supplied was equal to or less than the theoretical amount needed, cuprous ferrite (Cu2O·Fe2O3) was formed. As oxygen was increased to 10 percent excess and above, cupric ferrite (CuO·Fe2O3) was formed, and a mixture of the two forms was found when the excess oxygen in the fluidizing gas was 0 to 10 percent. The cupric ferrite proved easier to reduce, and to obtain maximum quantities of this form, the excess oxygen had to be above 10 percent but below 20 percent. Above 20 percent, excessive amounts of sulfur trioxides were formed which caused the bed to become sticky and hard to fluidize. When SO2 gas was used in the fluidizing gas, oxygen excess had to be kept at a minimum to prevent reaction (A-6) from shifting to the right, thus having the same effect as excessive oxygen with moderate concentration of SO2 gas,
SO2 + ½ O2 ↔ SO3……………………………………………………………………………..(A-6)
When the oxidized calcine was reduced with a simulated producer gas, the cupric ferrites reacted with hydrogen and carbon monoxide as follows:
3CuO·Fe2O3 + 4H2 ↔ 3Cu + 2Fe3O4 + 4H2O……………………………………………………..(A-7)
and 3CuO·Fe2O3 + 4CO ↔ 3Cu + 2Fe3O4 + 4 CO2………………………………………………(A-8)
In the case of the cuprous ferrite, the reactions are quite similar, being:
3Cu2O·Fe2O3 + 4H2 ↔ 6Cu + 2Fe3O4 + 4H2O……………………………………………………(A-9)
and 3Cu2O·Fe2O3 + 4CO ↔ 6Cu + 2Fe3O4 + 4 CO2……………………………………………(A-10)
Gas analysis on batch fluid-bed tests of the offgas showed that when reduction of the ferrites neared completion that hydrogen reduced CO2 by the reaction:
CO2 + H2 ↔ CO + H2O………………………………………………………………….(A-11)
The extent of this reaction during the reduction of ferrites in the continuous system, however, is not known.
Leaching
The rate at which metallic copper dissolves in aqueous ammonia solutions was found by F. Habashi to be represented by the equation:
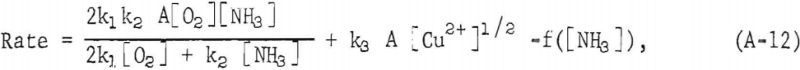
where k1, k2, k3 and f are constants, A is the surface area of the metal, and [O2], [NH3], [Cu²+] are oxygen, ammonia, and cupric ion concentrations, respectively. From this data, k1 was found to be 2.54 x 10-² cm sec -¹ , and
k2 = 2k1/280 = 1.81 x 10 -4 cm sec-¹. The first term in the rate equation is dependent upon the anodic and cathodic reactions:
Cu + 4NH3 → Cu(NH3)4++ + 2e……………………………………………………….(A-13)
and ½ O2 + H2O + 2e → 2 OH-…………………………………………………………(A-14)
Because Halpern proved that increasing OH ions decreased the rate, and since OH ion concentrations are a function of NH3 concentration, Habashi empirically corrected the rate equation A-12 by the term -f([NH3]). The k3
term is a function of the additional reactions:
Cu(NH3)4++ + Cu ↔ 2Cu(NH3)2+……………………………………………………….(A-15)
and Cu(NH3 )2+ + 2NH3 → Cu(NH3)4++ + e-……………………………………..(A-16)
Under conditions where the ammonia concentration is high, oxygen concentration is low, and Cu++ ion is present, the rate equation approaches:
Rate = k3 A[Cu++]½………………………………………………………………………(A-17)
In batch tests, it was found in the first 20 minutes, oxygen was not supplied to the solution fast enough to prevent a portion of the copper being converted from cupric to cuprous as per equation A-15. However, after 30 minutes, all the copper was oxidized back to the cupric ion by reactions A-14 and A-16. Because cupric ion in the leach solution was present in a quantity just sufficient to leach the copper from the reduced calcine, and a rapid depletion of cupric ion occurred early while nearly 85 percent of the metallic copper was dissolved, it is believed that reactions A-15 and A-16 play an important role in the leaching of the reduced calcine.
Solvent Extraction and Electrowinning
Solvent extraction of copper from ammoniacal solutions using LIX 64N has been investigated by General Mills, Inc., who found that copper exchanges for hydrogen in the organic according to the following equation:
Cu(NH3)4++ + SO4= + 2RH + 2H2O ↔ R2 Cu + (NH4 )2SO4 + 2NH4OH……………………….(A-18)
Copper is stripped from the organic in a similar manner as shown by
R2Cu + 2 (H+) + (SO4=) → 2RH + CuSO4………………………………………………..(A-19)
The cycle is, therefore, pH dependent with the capacity of the organic to hold copper decreasing with increasing hydrogen ion content. It also is evident that on loading the organic, ammonium sulfate and hydroxide needed for leaching are generated. Loss of ammonia is due mainly to evaporation and entrainment.
The chemistry of electrowinning of copper from sulfate solutions is well known. Copper is deposited and sulfuric acid regenerated by the reaction (11),
CuSO4 + H2O → Cu° + H2SO4 + ½ O2…………………………………………………(A-20)
Appendix B Equipment and Procedures
Fluid-Bed Roasting
Equipment and Operation
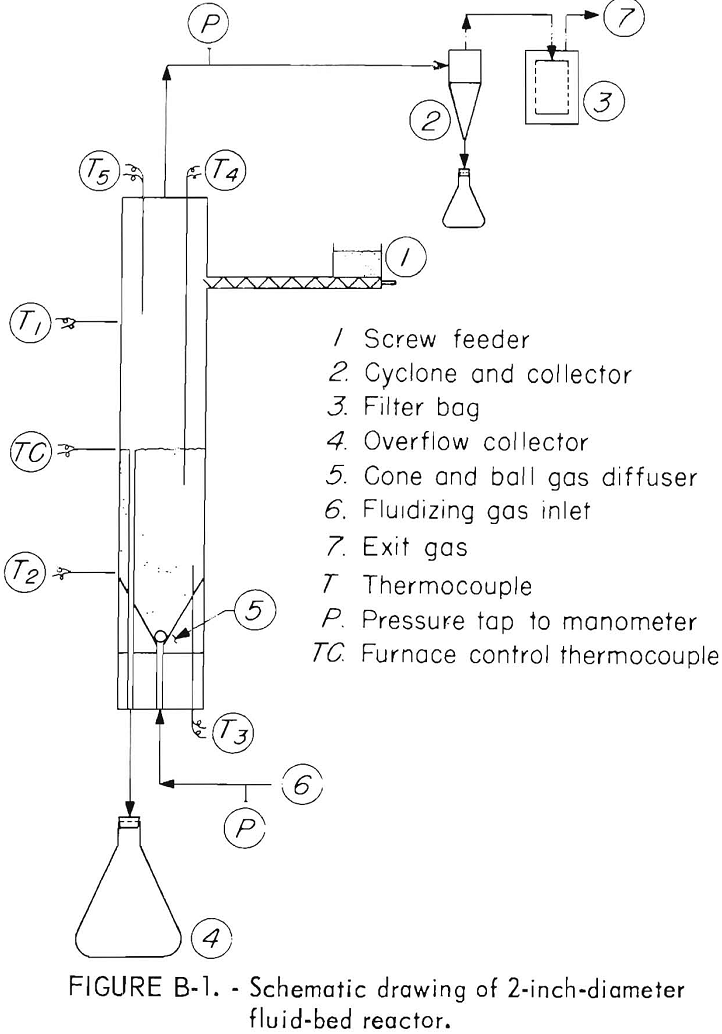
Continuous and batch fluid-bed oxidation and reduction tests were run in a 2-inch ID laboratory reactor. A schematic drawing of the reactor is shown in figure B-1. The reactor tube, constructed from type 316 stainless steel pipe, was 35 inches long from the cone and ball fluidizing gas diffuser to the top of the freeboard area. The reactor was heated in a 2-foot long, three-zone, automatically controlled electric tube furnace. Temperatures were recorded from thermocouples placed as shown in the diagram. Thermocouples T1 and T2 passed through the walls of the furnace and measured the temperatures of the outside wall of the reactor. Thermocouple T3 placed in a thermowell at the bottom of the reactor recorded the bottom bed temperature. Thermocouples T4 and T5 in thermowells protruding down through the top of the reactor measured temperatures at the top of bed and in the freeboard area.
The prepared concentrate or oxidized calcine was fed into the reactor 6 inches below the top using a screw feeder controlled by a variable speed motor. Bed height was established by varying the length of the ¼-inch-diameter overflow pipe which passed through the cone bottom gas diffuser to a collecting flask. Offgases passed through a 0.9 inch ID cyclone and then through a woven glass filter bag to remove entrained solids.
In each test, the reactor was charged with a bed of calcines from a previous test and heated to within 50° to 75° C of the operating temperature using nitrogen as the fluidizing gas. The gas composition
then was changed to the desired oxidizing or reducing proportions and the feed started. Enough feed was fed to the reactor to theoretically displace the bed twice. During this time, feed rate and reactor temperature were adjusted to the test conditions. Test samples were collected during one to two bed displacements. After samples of reactor overflow, cyclone, filter bag dust, and offgas had been taken, the feed was stopped and the reactor cooled using nitrogen as the fluidizing gas.
Mixtures of sulfur dioxide, oxygen, and nitrogen were used as the inlet fluidizing gas in the oxidizing fluid-bed roasting tests. The gases were metered through flowrators to provide a constant oxygen concentration of 21 percent, while sulfur dioxide was varied from 0 to 70 percent. The remainder of the inlet fluidizing gas was nitrogen. Two gas mixtures were used in the reduction of the oxidized calcine depending on whether the test was a batch or a continuous fluid-bed roast. In the continuous fluid-bed roast, the inlet fluidizing gas was a synthetic producer gas and analyzed, in percent, 12.5 H2, 20.5 CO, 3.0 CH4, 7.5 CO2, and 56.5 N2, and resulted in an offgas whose average analysis was, in percent, 3.9 H2, 13.8 CO, 12.3 CO2, and 70.0 N2. This latter gas composition was used in the batch tests so that in both types of fluid-bed tests, the solids were allowed to come to a steady state with the same gas composition. Gas analyses were performed with a gas chromatograph.
Standard Leach Tests for Oxidized Roast Products
Samples from the oxidation roasts were leached in water and in 1 M H2SO4 for 1 hour at 85° C at 16 percent solids. Leached residues and leach liquors were analyzed for copper and iron. The hot-water leach determined the amounts of copper and iron in the form of water soluble sulfates in the calcines. The 1 M H2SO4 leach indicated the total amount of copper and iron sulfates, including the complex oxysulfates and copper oxides. Some of the copper ferrite formed during the oxidation roast also appeared to be acid soluble under the conditions of the sulfuric acid leach.
Standard Leach Tests for Reduced Roast Products
The reduced products were agitation leached for 3 hours at room temperatures in a cupric ammonium carbonate solution which analyzed, in grams per liter, 20 Cu, 70 NH3, and 44 CO2. In all tests, an 18-gram sample was leached in 150 ml of the solution. Analyses of the liquor were made for copper by the Murexide-EDTA method, and copper extraction was equated to the metallic copper content of the sample. All residues were water washed, vacuum filtered, dried, and assayed.
Horizontal Batch Reduction Roast
Equipment and Procedures
Batch boat roasts were run using the same three-zone tube furnace and control used for the fluid-bed tests. The furnace was rotated to the horizontal position and the fluid-bed reactor replaced with a 3-foot long, 64-mm diameter Vycor tube. Rubber stoppers protected by asbestos disks were used to seal both ends of the tube. One stopper contained a gas inlet tube and a Vycor thermocouple well which extended 18 inches to the center of the furnace. The second stopper contained a gas outlet tube. The temperature on the inside and from two places on the outside of the tube were recorded. Gases exiting the tube were passed through a water seal before exhausting into the hood.
In each test, 40 grams of the bulk oxidized calcine was placed in a ceramic boat 6-inches long by 1-inch wide by 1-inch deep, and produced a depth of charge of one-half of an inch. The charged ceramic boat was placed in the Vycor tube at the center of the furnace, the ends of the tube were sealed, nitrogen gas was flushed through to remove the oxygen, and the furnace was heated to the desired test temperature. When the temperature was stabilized, the sweep gas was changed to the fluid-bed offgas composition which analyzed, in percent, 3.9 H2, 13.8 CO, 12.3 CO2, and 70.0 N2. At the end of the test time, the sweep gas composition was changed to nitrogen, and the tube containing the ceramic boat was removed from the furnace and allowed to cool. The reduced calcine was mixed, assayed, and subjected to the standard ammoniacal leach test as described in section I of this appendix.
Leaching
Batch Tests
Agitation leaching was done in a 1-liter beaker containing 500 milliliter of ammoniacal sulfate or carbonate leach solution containing variable amounts of dissolved cupric ion and variable weights of the bulk reduced calcine. Air was bubbled through the solution at a rate of 150 cubic centimeter per minute. Solution samples of 1 milliliter each were taken at time zero, every 5 minutes thereafter for 30 minutes, and at 45, 60, 90, 120, and 180 minutes. Analyses for copper was done by the Murexide-EDTA titration method.
Continuous Tests
A three-stage agitation system was constructed out of 1-liter beakers with overflows attached to the beakers at the 600 milliliter mark. Solution and solids were fed concurrently at 4.5 percent solids at a rate to give 1.5 hours retention time. Air was bubbled into each of the three stages, and each was agitated by a variable speed motor. The overflow from the third stage was allowed to settle, the solution was decanted, and the remainder was filtered on a Buchner funnel. Samples were analyzed by conventional methods.
Solvent Extraction and Electrowinning
Continuous solvent extraction equipment consisted of four mixer-settler units. Each unit contained a mixer made of a 400-milliliter electrolytic glass beaker and an 800-milliliter glass beaker settler. The mixer-settler units were connected by plastic tubing resulting in a mixer volume of near 300 milliliters and a settler volume of near 600 milliliters. The feed rate to the loading circuit was 21 milliliters per minute of organic and 7 milliliters per minute of aqueous, resulting in a retention time in each mixer of 10 minutes and 0.07 milliliter per minute per square centimeter total settling area. Aqueous feed rate to the stripping cells was 21 milliliters per minute which gave a mixer retention time of 7 minutes and 0.1 milliliter per minute per square centimeter total settling area. The aqueous from the final stripping cell was aerated to remove any entrained organic and then fed to the electrolytic cell where copper was removed and sulfuric acid generated.
The electrolytic cell was constructed of clear plastic and measured 10 by 8.7 centimeters and was 14 centimeters deep. The cell contained two lead anodes and one copper cathode each having an effective surface area of 162.2 square centimeters. Stirring within the cell was accomplished by pumping the cell electrolyte at about one displacement every 2 minutes in such a fashion that the solution was streamed past both surfaces of the cathode. Cell power was 5.3 amperes at 2.5 volts.
Appendix C Product Description and Feed Preparation
Chalcopyrite Flotation Concentrate
Chemical Analysis and Microscopic Examination
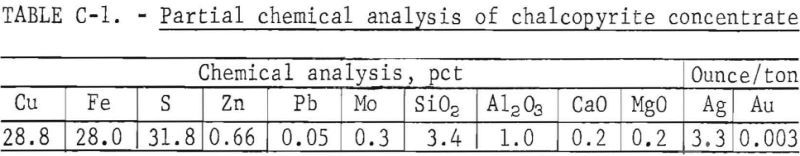
A copper sulfide concentrate generally typical of those produced by flotation of porphyry copper ores was used in the investigation. Partial chemical analysis of the sample is given in table C-1, and a wet screen analysis is provided in table C-2.
Microscopic examination of polished briquette surfaces revealed that the concentrate was composed predominantly of chalcopyrite with minor amounts of covellite, chalcocite, quartz, orthoclase, calcite, and talc. Sporadic grains of sphalerite, pyrite, metallic copper, molybdenite, and metallic iron contaminant from grinding also were observed.
Most of the chalcopyrite was contained in the minus 200-mesh sample. The only minerals interlocked with chalcopyrite were chalcocite and covellite. Many of the pyrite grains were rimmed with a thin coating of covellite. The gangue minerals, such as quartz and feldspar, contained inclusions of chalcopyrite. Most of the silicate gangue occurred in the plus 325-mesh fraction.
Concentrate Preparation for Oxidation Roast Feed
The fine chalcopyrite flotation concentrate required pelletization to reduce dusting in the reactor. Pellets were prepared by adding 200 milliliters of 5 percent H2SO4 to a 1-kg charge of chalcopyrite flotation concentrate and mixing in a 20-inch-diameter bowl-shaped pelletizer. The balled material was sized and dried at 105° C to provide minus 20- plus 65-mesh pellets for reactor feed. Silica sand was added to the concentrate to abrade coatings that tended to form on the reactor walls. The high purity, smooth, spherical-grained sand was essentially all minus 48-plus 150-mesh size. An
amount equivalent to 10 percent by weight of the total charge was mixed with the pelletized concentrate before feeding to the reactor.
The acid pellets were used during oxidation roast testing in the 2-inch fluid-bed reactor, but they proved to be too soft in larger fluid bed tests. Therefore, to produce a hard pellet, bentonite was added in the amount of 2 percent of the feed weight. These were used for the material balance oxidation roast and the production of large quantities of oxide calcine for reduction and leach studies.
Oxidized Calcine Description
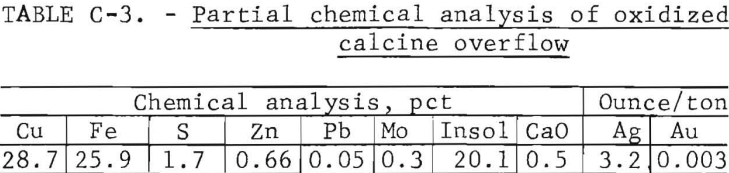
A partial chemical analysis for the bulk overflow calcine collected from continuous fluid-bed oxidation roasts run at the optimum operating conditions is provided in table C-3. The calcine was used without further treatment in the reduction roast studies. Dry screen analysis of the calcine showed 69 percent to be plus 100-mesh and 20 percent minus 200-mesh. Microscopic examination of polished briquette surfaces revealed that the oxidized calcine was composed mostly of cupric ferrite and silica with minor amounts of chalcocite, chalcopyrite, and orthoclase. The chalcopyrite grains during oxidation roasting were partially altered, as shown in figure C-1, going from a CuFeS2 center to a Cu2S rim. The majority of the material is composed of a lacy-structured pellet of ferrite.
The oxidized filter bag dust had a partial chemical analysis of, in percent, 19.3 Cu, 24.2 Fe, and 11.4 S. Nearly half of the copper was water soluble sulfate and 79 percent was acid soluble. Thus, the dust from oxidation roasting would need to be recycled to the pelletizer and then reoxidized.
Bulk Reduced Calcine Description
A partial chemical analysis of the bulk reduced calcine made by batch fluid-bed roasting of the oxidized calcine is shown in table C-4.
Reduction roasting conditions were 800° C, a retention time of 3 hours, and the reducing-fluidizing gas assayed, in percent, 3.9 H2 , 13.8 CO, 12.3 CO2, and 70.0 N2. The reduced calcine was used without further treatment for leach tests, and by dry screen analysis was 46 percent plus 100 mesh and 34 percent minus 200 mesh.
Microscopic examination of polished briquette surfaces revealed that the calcine contained major quantities of metallic copper, iron oxides, and silica. Minor quantities of chalcocite, pyrite, and orthoclase also were present. The particles of metallic copper were usually less than 10 microns in diameter and were loosely held together in the pellet by an iron oxide matrix as shown in figure C-2. The near white material is metallic copper and the light gray materials are iron oxides.
The reduced filter bag dust had a partial chemical analysis of, in percent, 46.7 Cu, 43.0 Fe, 0.77 S, and 3.0 SiO2, and the standard ammoniacal leach extracted only 62.1 percent of the copper. In the completed process, this dust would be recycled to the pelletizer.
Ammoniacal Leach Residue Description
Table C-5 is a partial chemical analysis of the residue obtained by continuous agitation leaching of the bulk reduced fluid-bed calcine followed by a water wash and vacuum filtration. Microscopic examination showed that (1) metallic copper was absent; (2) pyrite grains averaged about 200 mesh in size; and (3) the major source of copper was chalcocite and chalcopyrite.
Appendix D Oxidation Roast-Material Balance-and-Cost Data
Oxidation Roasting of Chalcopyrite
The data given here were presented at the February 1972 Annual AIME Meeting in San Francisco, Calif., and describe the effect of time, temperature, and SO2 content of the fluidizing gas on the oxidation of chalcopyrite concentrate as shown by the solubility of the calcine In water and sulfuric acid.
Influence of Retention Time
Retention time of the chalcopyrite pellets in the reactor was varied from 0.4 to 3 hours by varying the length of the overflow pipe. Tests were run at temperatures of 700° and 800° C using an inlet fluidizing gas of either air (0 percent SO2), or a gas containing, in percent, 25 SO2, 21 O2 , and 54 N2.
The influence of retention time on acid and water solubility of the resultant calcines is shown in figure D-1.
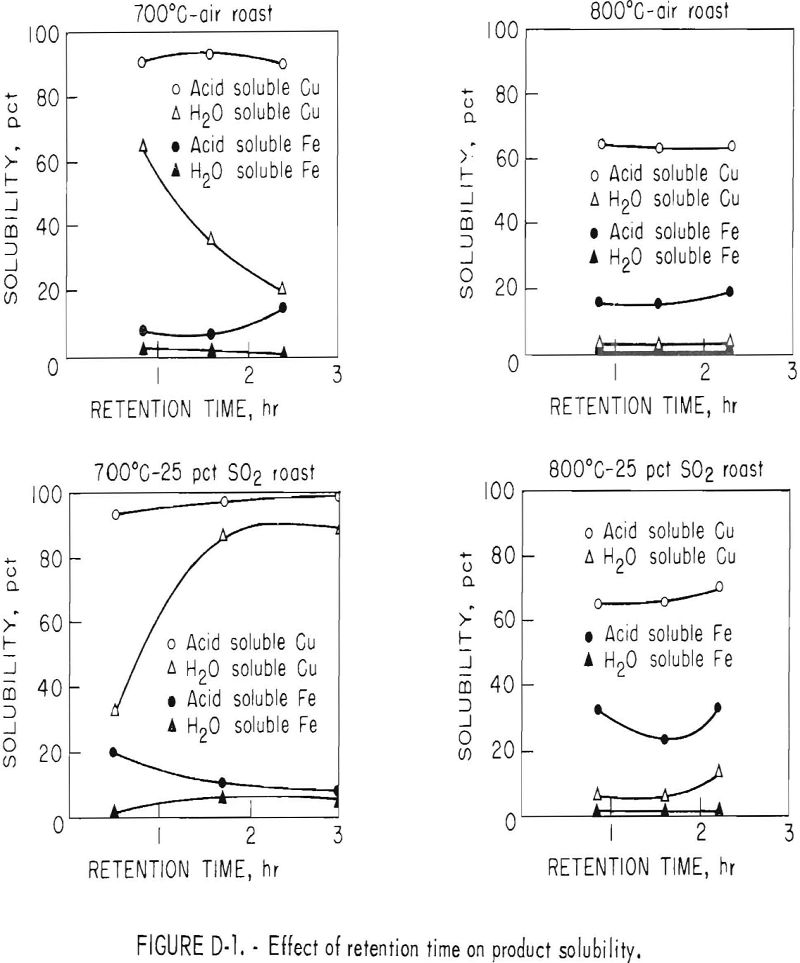
The curves show that water soluble copper or copper sulfate fraction was most affected by time in the 700° C series. In the air oxidation roast, water soluble copper decreased from 65 to 20 percent as retention time was increased from 0.9 to 2.5 hours. Prolonged roasting in dilute SO2 gas caused the soluble copper sulfate initially formed to partially decompose. With 25 percent SO2 in the fluidizing gas, the partial pressure of the sulfur dioxide prevented the decomposition reaction from taking place. Water soluble copper increased from 33 to 89 percent as retention time was increased from 0.4 to 3 hours.
When the roasting temperature was increased to 800° C in either of the fluidizing gases, most of the copper sulfate decomposed to water-insoluble copper oxides and ferrites, Even with 25 percent SO2, the temperature effect was predominant, causing copper sulfate to decompose to copper oxide.
The curves also shows that oxidation at 800° C at times greater than 0.9 hour did not appreciably effect the copper solubility in acid. However, total acid-soluble copper dropped from a range of 90 to 97 percent to a range of 60 to 70 percent when the roasting temperature was increased from 700° to 800° C. At the lower temperature, copper in the calcine was almost entirely in the form of copper sulfate and oxide, whereas copper ferrite was produced at the higher temperature even when roasting in the presence of sulfur dioxide. Residual copper sulfide never exceeded 3 percent.
Iron solubility curves, also shown in figure D-1, are more complex than the copper curves. In general, the minimum acid-soluble iron was obtained at about 1.5 hours retention time when roasting at either 700° or 800° C. Acid solubility of iron tended to increase or remain constant in all tests in which retention time was longer than 1.5 hours. Two reactions are believed to be responsible for the concave-shaped curves. First, the initial decrease in acid-soluble iron was caused by the decomposition of soluble sulfates and oxysulfates to insoluble iron oxides. The iron oxide then reacts with copper oxide to form copper ferrite. Increase in iron solubility with time is probably caused by a change from insoluble iron oxide to a ferrite somewhat soluble in acid.
Influence of Temperature
Tests were made varying the furnace temperature from 600° to 800° C while roasting chalcopyrite in air and SO2 atmosphere. In this test series, the fluidizing gases were air; a gas containing 25 percent SO2, 21 percent O2, and 54 percent N2; and a gas containing 70 percent SO2, 21 percent O2, and 9 percent N2. The influence of temperature on acid and water solubility of the calcines is shown in figure D-2.
The amount of copper sulfate in the calcines, as represented by the water-soluble copper, decreased with increasing temperatures. However, as the SO2 concentration of the fluidizing gas was increased, the temperature at which the copper sulfate decomposed to copper oxide increased, resulting in a shifting of the curves to the right.
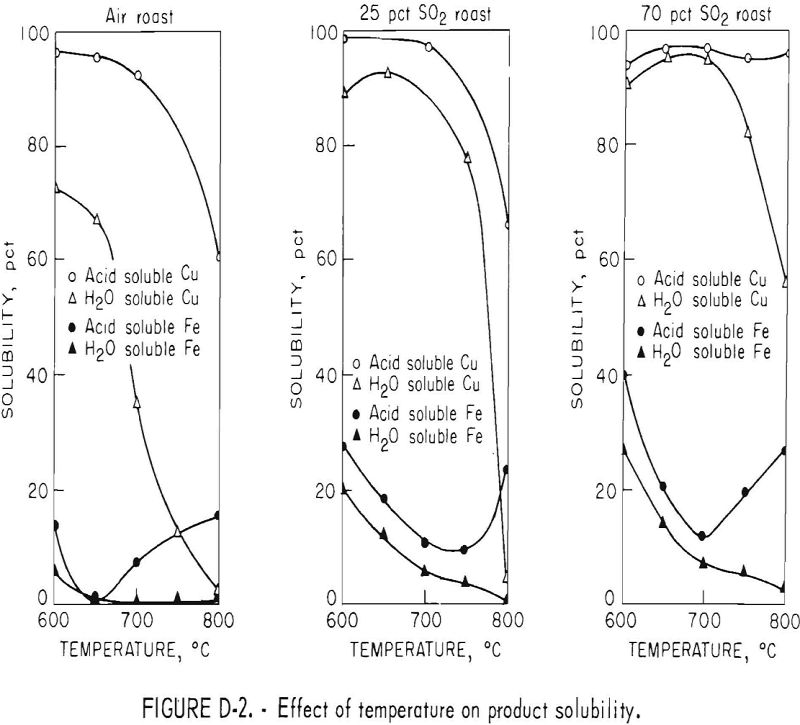
The total acid-soluble copper content of the calcines decreased with increased temperature because of the formation of copper ferrites, which begins to take place at 700° C when roasting in air. The higher concentrations of sulfur dioxide in the fluidizing gas did increase the temperature at which the ferrites formed, because the rate of decomposition of copper sulfate to the oxide was slowed and copper has to be in the oxide form before ferrites can be produced. The overall effect of increasing temperature was similar to that of increasing retention time in that copper sulfate was decomposed to oxides and then altered to ferrite. The addition of sulfur dioxide to the inlet fluidizing gas tended to counteract the temperature effects, resulting in a decrease in ferrite formation. The 95-percent acid-soluble copper figure obtained for the 800° C, 70-percent SO2 roast would indicate that ferrite formation was inhibited. However, the water-soluble copper content decreased to 56 percent and iron solubility increased indicating that ferrites were beginning to form.
Water-soluble iron content of the calcines decreased as roast temperature was increased at all SO2 concentrations of the inlet fluidizing gas. However, much more water-soluble iron was present in calcines produced at low temperatures in the 70-percent SO2 gas than in the air roast. Total acid-soluble iron decreased as the temperature was increased, reaching a minimum at about 700° C, then increased with rising temperature. As in the case with retention time, the acid-soluble iron sulfates initially decomposed to acid-insoluble oxides, but as the temperature increased, copper ferrites formed. As a result, the acid-soluble iron content of the calcines increased, but water-soluble iron continued to decrease.
Influence of Sulfur Dioxide Concentration of Fluidizing Gas
The effect of sulfur dioxide in the inlet fluidizing gas on the roasting of chalcopyrite is shown in figure D-3. These curves were obtained by replotting the temperature test series results in terms of solubility versus sulfur dioxide concentration at constant temperatures of 600°, 650°, 700°, 750°, and 800° C.
The curves show that as the SO2 concentration increased, the water-soluble copper sulfate content of the calcine increased at all temperatures. The total acid-soluble copper remained fairly constant in the 90 to 97 percent range at temperatures of 600° to 700° C because copper ferrite formation had not begun. At temperatures of 750° and 800° C, acid-soluble copper was only 60 to 70 percent with no sulfur dioxide in the fluidizing gas, but it increased rapidly as the SO2 concentration was increased.
Water-soluble iron increased as the SO2 concentration in the inlet fluidizing gas was increased, but the amount of water-soluble iron decreased
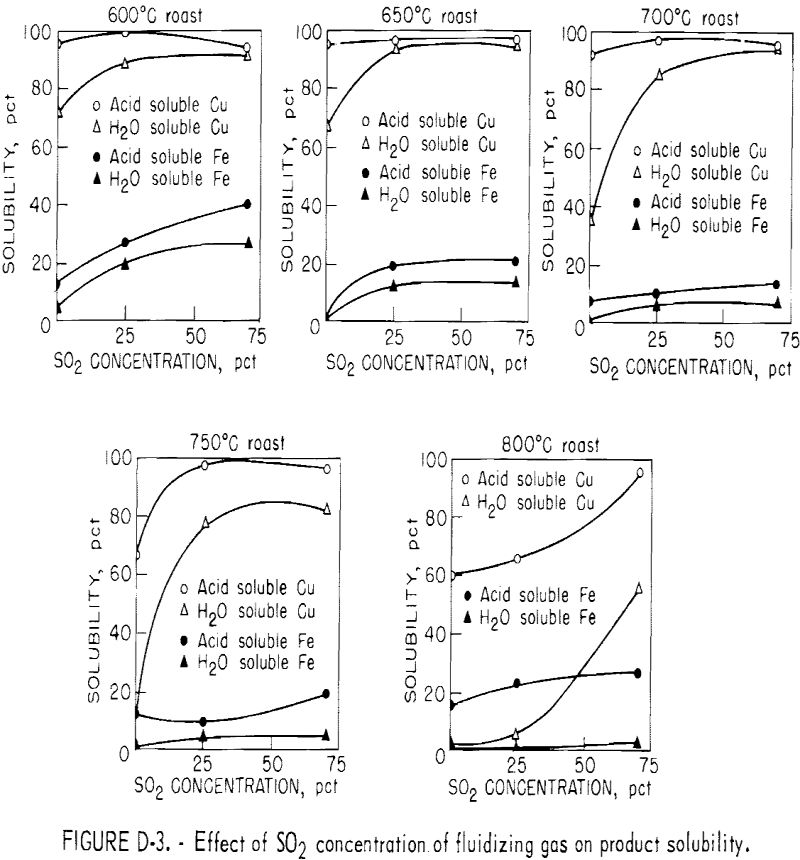
as the temperature increased, owing to decomposition of the iron sulfates to oxides. Acid-soluble iron also increased with SO2 concentration at temperatures of 600° to 700° C because of a decrease in the decomposition of iron sulfates and oxysulfates to oxides. At 750° C, an initial decrease in the acid-soluble iron was observed as the SO2 concentration increased. This was attributed to the suppression of the formation of copper ferrites.
Material Balance
Calculations for the material balance are based upon 1 ton of chalcopyrite concentrate fed to the pelletizer with 40 pounds of bentonite, and the addition after pelletization of 222 pounds of sand.
The oxidation fluid-bed roaster was run continuously with the cyclone dust recycled, but the filter bag dust was not recycled. The products from oxidation are shown in table D-2. In an overall process, all dust would be recycled, and the overflow would be the only solid product from the roaster. Therefore, even though the filter bag dust and the reactor cleanout are listed here as products from the oxidation roast, they would not be considered as sources of copper loss. The equilibrium dust loading in the roaster gas and its influence on cyclone, baghouse, and roaster operation would have to be determined by large-scale continuous tests.
The oxidized calcine was batch fluid-bed reduced with the cyclone dust being recycled back into the bed and the filter bag dust and the bed products collected. The filter bag dust would be recycled in a continuous process. As with the oxidizing roast, large-scale continuous testing with dust recycle would be needed to determine equilibrium dust loadings and the effect of recycled dust on roaster and dust collector operation. The products produced and the material balances obtained by the reduction of the 1,866 pounds of oxidized calcine (overflow table D-2) are shown in table D-3. The offgas contained 25 pounds of sulfur per ton of concentrate treated which represents about 4 percent of the total sulfur and would need to be treated for sulfur dioxide removal. Oxidation roasting on a larger scale should reduce the short circuiting observed in the 2-inch-diameter reactor and, therefore, reduce the sulfur emitted in the offgas during reduction roasting. However, larger scale testing is needed to determine the quantity of sulfur that would actually be present in the reduction roast offgas for a commercial operation.
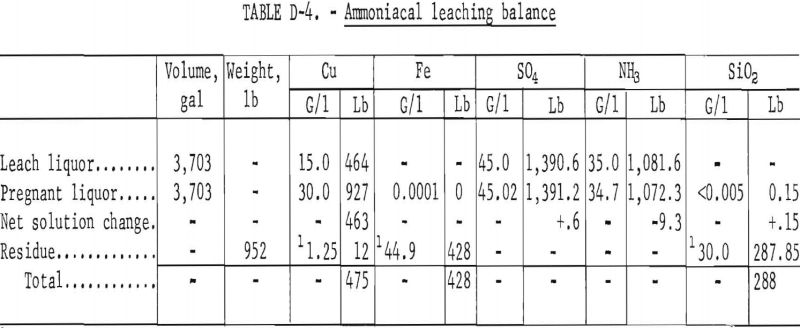
Leaching of the reduced bed (overflow) product was done in a three-stage concurrent agitation leach at ambient conditions using a solution of cupric ammonium sulfate. A material balance of the procedure based on the feed of 1,454 pounds of reduced bed product is shown in table D-4. The residue of the leach was washed with water and, therefore, losses of copper and ammonia by solution entrained in the solids are not shown. Cost evaluation was based on losses in previous commercially used steam washing circuits.
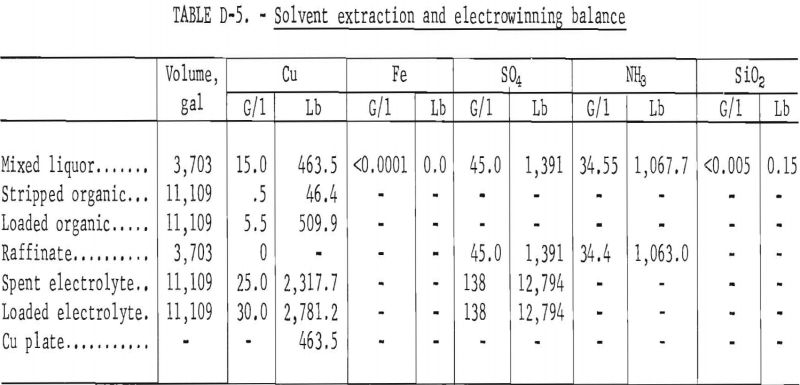
Pregnant liquor was diluted one to one with raffinate from the solvent extraction circuit which reduced the copper content to 15 grams per liter. The concentrations of ammonia and sulfate remained nearly the same. One-half of the mixed liquors (3,703 gallons) were fed to the solvent extraction-electrowinning circuit. A balance of the circuit is shown in table D-5.
The other 3,703 gallons of mixed feed liquor after being adjusted to 35 grams ammonia per liter were used for leaching the reduced calcines. Ammonia losses from table D-4 and D-5 appear to be 9.3 pounds during leaching and 4.7 pounds during solvent extraction. However, in a continuous process where the filter dusts are recycled, the total calculated residue weight would increase from 952 to 1,106 pounds. Ammonia losses, therefore, during leaching would increase to 11 pounds and solvent extraction losses would increase to 6 pounds for a total ammonia loss of 17 pounds per ton of concentrate treated.
In addition, aqueous solution containing ammonia is entrained in the organic and transferred to the electrowinning circuit. Ammonia will build up in the electrolyte to a value of nearly 30 grams per liter before reaching an equilibrium with the transfer of aqueous strip to the extraction circuit.
Cost Evaluation
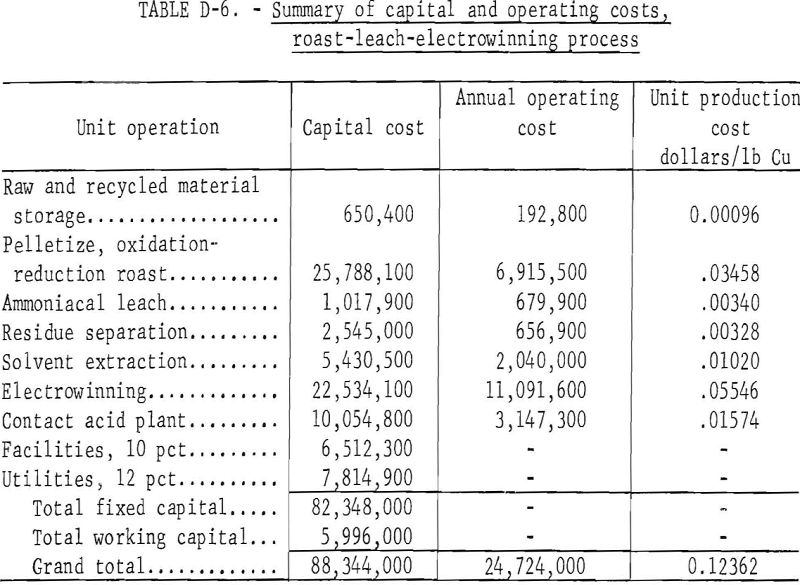
The cost evaluation, based on laboratory studies, was for a plant treating 1,000 tons per day of copper concentrates. The laboratory work required the addition of sand to the roaster feed to inhibit scaling. For the evaluation, it was assumed that no sand would be needed in the production size roasters. In addition, it was assumed that the filter bag dusts could be returned to the feed pelletizer without causing a dust buildup. Costs for steam washing and the resultant copper and ammonia losses were calculated from data reported in the literature. A flowsheet for the process is shown in figure D-4.
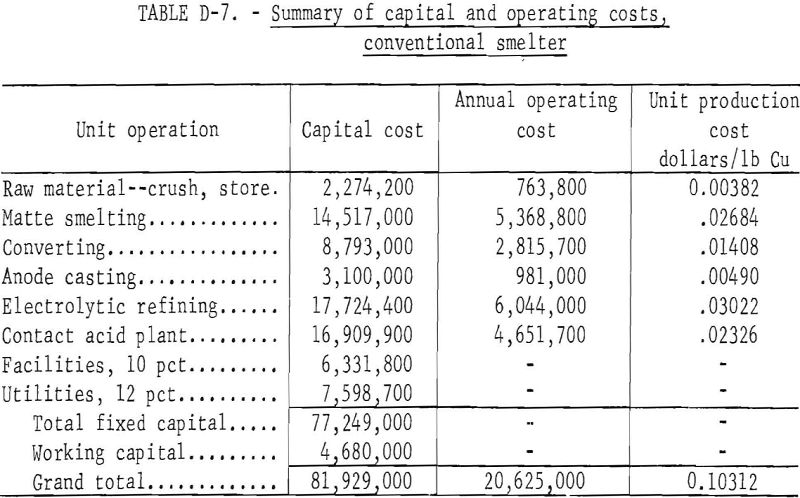
Capital costs were determined by standard chemical engineering cost estimating procedures and from published information. The interest rate during construction was taken at 8 percent. All costs were based on 1973 equipment cost indexes and labor rates. Operating costs assumed direct labor at $4.45 per hour, electric power at $0.01 per kilowatt-hour, natural gas at $0.50 per thousand cubic feet, steam at $0.34 per thousand pounds, ammonia at $60 per ton, process water at $0.17 per thousand gallons, cooling water at $0.03 per thousand gallons, and coal at $12 per ton. Other assumptions are supervision of direct labor and maintenance labor at 15 and 20 percent, respectively; maintenance labor at 2.3 percent of fixed capital cost without interest; payroll overhead at 25 percent of total labor and supervision; operating supplies at 20 percent of the cost of maintenance; administration and overhead at 40 percent of the total cost for direct labor, maintenance, and operating supplies; taxes and insurance at 2 percent of the fixed capital cost without interest; an amortization period of 12.5 years; and 330 operating days per year. A summary of the capital and operating costs for the double roast process and for a conventional smelter are shown in tables D-6 and D-7, respectively. These costs are for comparative purposes only; they show that the double roast process costs more to build and to operate. The unit production cost per pound of copper of this process is about $0.0192 greater than for a conventional smelter. However, if consideration is given to the value of byproduct sulfuric acid, the double roast process would be competitive with conventional smelting of copper concentrate.