Iron Oxide Pellets
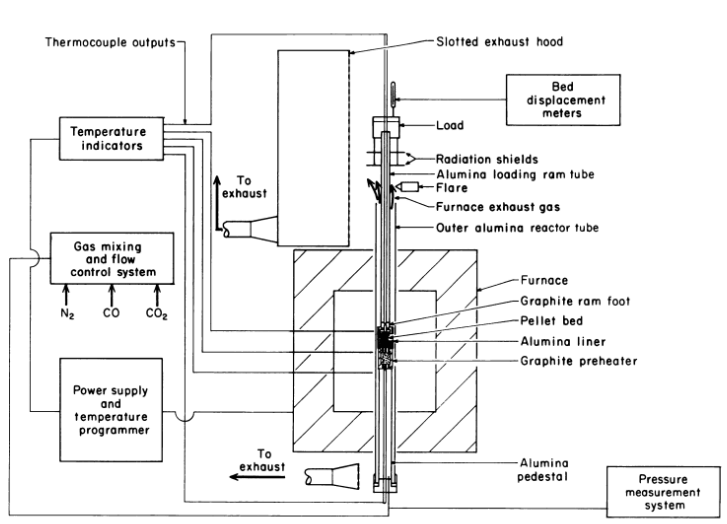
Develop methods of enhancing and measuring the high-temperature softening and melting properties of iron oxide pellets reduced under simulated blast furnace smelting conditions. Add dolomite and limestone flux and a low-cost organic binder, such as starch, carboxyl methylcellose (CMC), or waste papermill sludge, to the iron oxide concentrate to produce hematite (ferric oxide) pellets with […]
Zirconium Crucibles
Zirconium crucibles are rapidly becoming a standard piece of equipment, particularly in laboratories where fusions using strongly alkaline fluxes are common. This Center has found no better material to use for the thousands of sodium peroxide fusions that are done here every year. Zirconium crucibles usually arrive from their suppliers in a polished, silvery state. […]
Acid Digestion of Ferrochrome Metal
At times it may be necessary or desirable to dissolve a ferrochrome sample in acid rather than fusing it in sodium peroxide. Acid digestion methods are slow, but require little in the way of attention. Two alternative methods are described here. The A method is the basic method to use for most ferrochrome samples. The […]
Manganese in Chromite, and Ferrochrome
Manganese commonly occurs in mineral chromite as an impurity, and the amount can range from trace quantities to a few percent. In ferrochrome smelting operations, the manganese should find its way into the slag rather than the metal. Data from these analyses have most often been used to trace the partitioning of the manganese through […]
Gravimetric Silica in Chromite and Ferrochrome Slag
Silica is present in chromite ores as a basic silicate. In characterization and beneficiation studies performed on chromites at this Center, the chromium-silica ratio determines the purity of the gangue material being analyzed. Accurate characterization of the original ore material and the further feasibility of smelting to ferrochrome are identified by using this ratio. The […]
Aluminum and Magnesium in Chromite and Ferrochrome
Aluminum and magnesium occur as gangue components in chromite ores and ferrochrome slags. At this Center, ores from many different sites and of many varieties are examined for commercial feasibility. The beneficiation of these ores is a continuing activity that produces most of the samples that are analyzed with this method. Data from this method […]
Metallic Iron in Ferrochrome Slags
It would truly be unusual to find a sample of mineral chromite with metallic iron in it. However, (his Center has handled many samples of prereduced smelter charges with several percent of metallic iron and ferrochrome slags with measurable quantities of metallic iron. Therefore, this method finds its use in the later stages of mineral […]
Ferrous Iron in Mineral Chromite and Ferrochrome Slags
The determination of ferrous iron in mineral chromites and other chromite-bearing samples is limited by the difficult solubility of the chromite lattice. Reaction of the ferrous iron immediately upon release from the crystal seems to be the preferred way of measurement. Attempts to dissolve chromite by other methods depend on strong oxidizing agents that leave […]
Total Iron in Mineral Chromite and Ferrochrome Slags
Iron is the second major element in chromite. In smelting operations where ferrochrome is the product, a specific range in the chromium-to-iron ratio is desired. Where pure metallic chromium is the product, the iron concentration is not as important. In both cases, it is important to have a good value for the chromium-to-iron ratio. A […]
Acid-Soluble or “Metallic” Chromium in Ferrochrome Slags
At this Center, results from this method have been used almost exclusively as data for the calculation of the total degree of reduction in a smelter batch, rather than as an indicator for the metallization of chromium. When a charge of chromite material is smelted or prereduced, the crystal structure is changed, and once the […]
