OXFLOAT CTC3
The earlier versions of OXFLOAT CTC3 had been in production since 1972 and currently Oxflo’s manufacturing partner supplies 700 mt of this product to their local market annually. They have a further manufacturing capacity of 800 mt, taking them to a total of 1500 mt per year. Currently OXFLOAT CTC3 has displaced AM2 at one […]
Pb Oxide Floatability & Selectivity VS Sulphidizer Usage
The use of a sulphidizer is common in oxide flotation recovery. Na2S (Sodium Sulfide) can help “activate” oxide minerals behave as sulfides. The tests performed by both vendors showed noticeable technical differences between both reagents. AM2 constantly requires the use of Na2S as its copper activator while CTC3 does not rely on Na2S. This dependence […]
Naturally Floating Gold and Silver Lost in Pre-Flotation
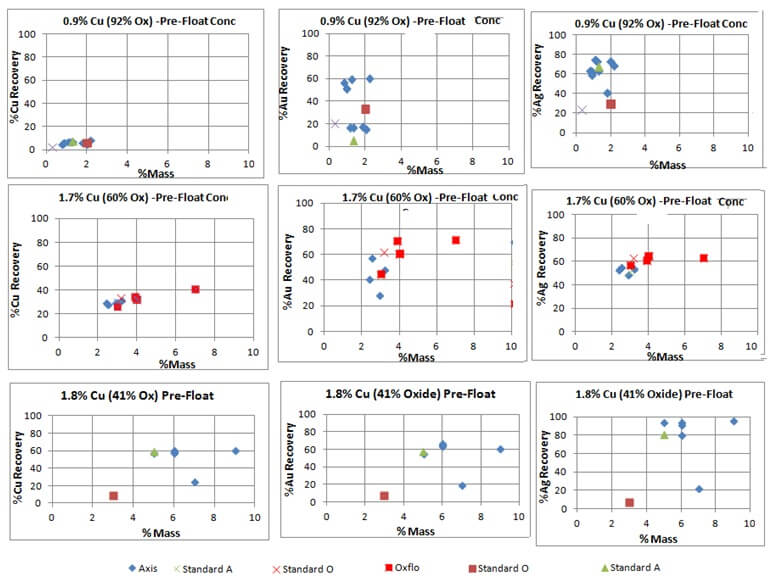
It is clear the Pre-Float stage creates much looses in Cu, Au and Ag. Mineralogy work on this stream would help understand why the metals are naturally floatable using only MIBC. Knowing the cause of this floatability (chemical, physical or mechanical) would open the opportunity to implement solutions that would increase Cu, Au and Ag […]
Copper Oxide Flotation
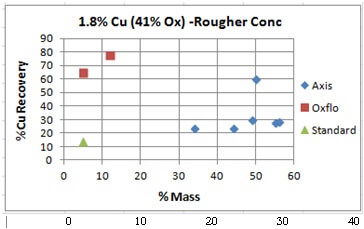
Continuing with the evaluation of copper oxide flotation using hydroxamate we now evaluate a rock that’s 41% copper oxide with some lead oxide included. Sample B: 1.8% Copper about 41% Oxide Cu Rougher Tests: Unseen in these charts is the order in which the results were obtained. The last/best “Blue” dot (the best data point) […]
Hydroxamate Collectors
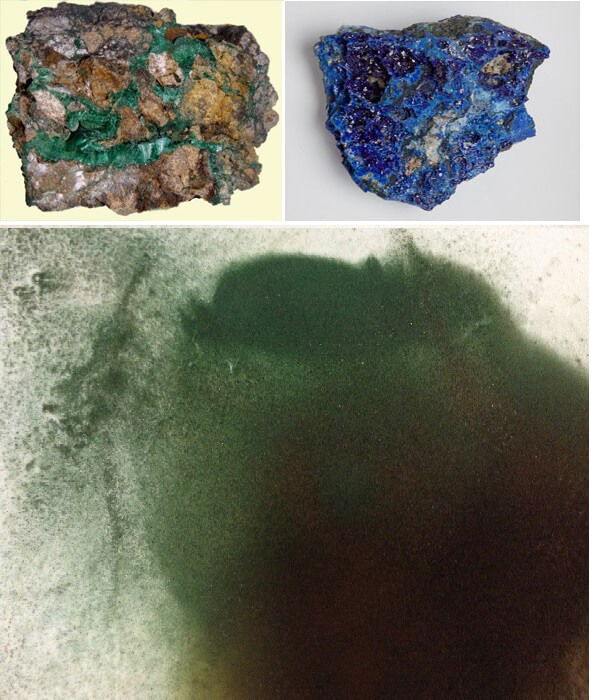
Let me point you to two good papers on the flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. A great detailed thesis called “An Investigation of Copper Recovery from a Sulphide-Oxide Ore with a Mixed Collector System” and one called “Flotation of mixed copper oxide and sulphide minerals with xanthate and […]
Benefits of using SART on a Zn CN solution
In the absence of SART Process, cyanide is consumed by the leaching of cyanide-soluble zinc minerals present in the ore, generating a WAD cyanide-zinc complex (Zn(CN)42-) in solution. This dissolved WAD CN-Zn complex circulates back to the leach in the recycled Barren Leach Solution (BLS), and further reacts with Ag2S in the fresh ore to […]
