Zinc Cyanide & Silver Gold Precipitation
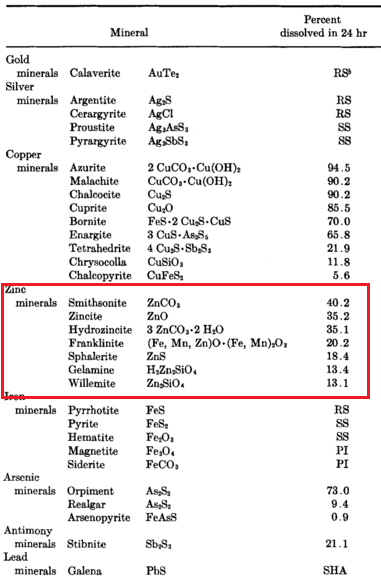
In cyanidation, zinc (the Merrill-Crowe Process) is practically the universal precipitant for precious metals dissolved in cyanide solutions although for specific reasons aluminium and charcoal have been used. During the reactions involved in precipitation, the greater part of the zinc dissolves in the cyanide solution and forms various cyanogen complexes such as sodium or calcium […]
Effect of Temperature on Gold Cyanidation

When heat is applied to a cyanide solution containing metallic gold, two opposing factors affect the rate of dissolution. The increase in temperature would be expected to increase the activity of the solution and thus increase the rate of dissolution of gold. At the same time the amount of oxygen in the solution would decrease […]
Effect of pH Alkalinity on Gold Leaching

The role pH has in affecting gold leaching rates by cyanide and the functions of calcium hydroxide in cyanidation are as follow: 1. For safety and to prevent loss of cyanide by hydrolysis. 2. To prevent loss of cyanide by the action of carbon dioxide in the air. 3. To decompose bicarbonates in mill water […]
Effect of Oxygen on the Dissolution of Gold

In discussing the effect of oxygen on the gold dissolution, one can understand the use of oxygen or an oxidizing agent is essential for the dissolution of gold under normal conditions of cyanidation. Such oxidizing agents as sodium peroxide, potassium permanganate, bromine, chlorine have been used with more or less success in the past; but […]
Gold & Silver Dissolution and Cyanide Concentration

By far the most important cyanides used in cyanidation are sodium cyanide and calcium cyanide. The latter product is sold in an impure form analyzing close to 50% NaCN equivalent. The former is sold in various grades from 85 to 98% NaCN. In comparing the dissolving effects of the cyanides of ammonium, sodium, potassium, magnesium, […]
Cyanide Leaching Chemistry & Gold Cyanidation
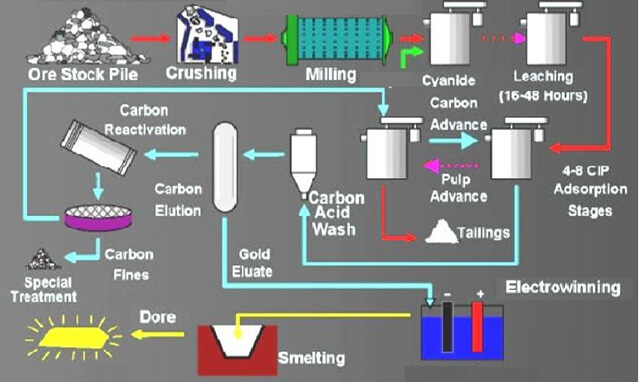
The reactions that take place during the dissolution of gold in cyanide solutions under normal conditions have been fairly definitely established. Most agree that the overall cyanide equation for leaching and cyanidation of gold is as follows: 4 Au + 8 NaCN + O2 + 2 H20 = 4 NaAu(CN)2 + 4 NaOH In a […]
Sequential Copper–Lead–Zinc Flotation
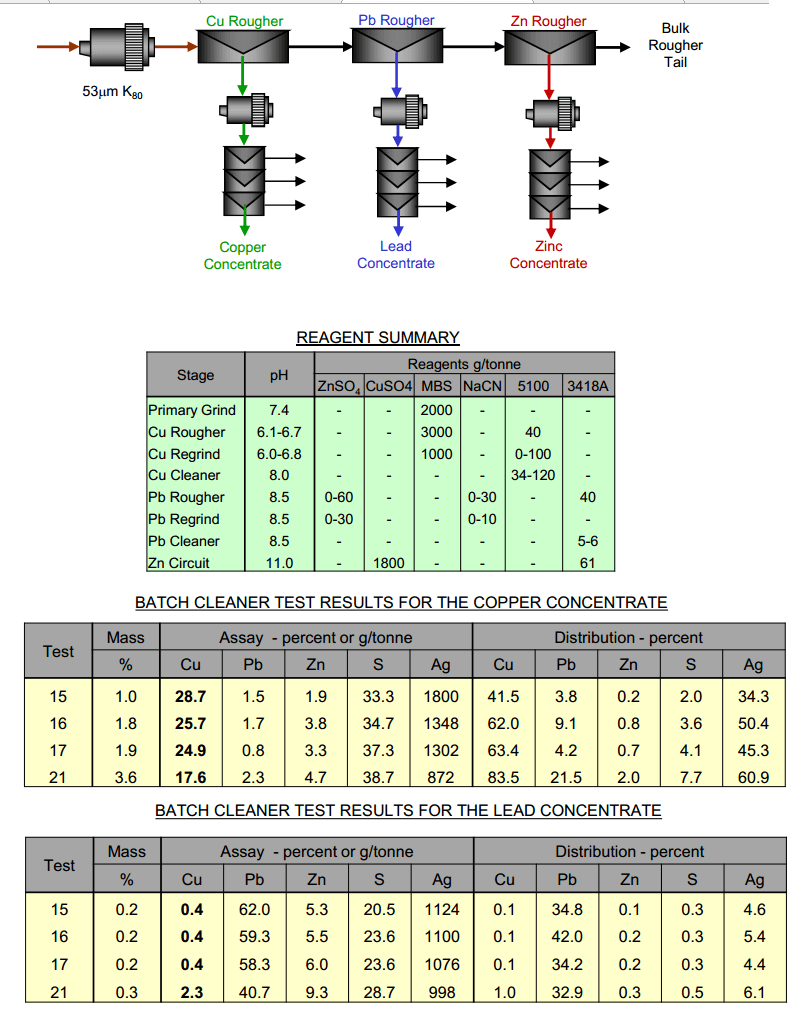
To improve the selectivity between copper and lead, a sequential copper-lead-zinc rougher circuit was tested. Each of the rougher circuits had a dedicated regrind and cleaner circuit. The advantages of the sequential circuits are that the levels of selectivity can be increased, particularly for copper and lead. With this process, copper was recovered first with MBS depression of lead, […]
Clarkson Liquid Reagent Feeders

Original Clarkson Feeder Clarkson Feeders Clarkson Reagent Feeder The Clarkson Company had once designed a combination drum and scoop type liquid reagent feeder, the largest size of which has a capacity rating of 2000 tons of dry ore per 24 hr. This will enable the feeder to take a large tonnage of initial feed and several […]
Arsenic and Antimony Sulphide Minerals in Cyanidation

The successful cyanidation of gold ores containing appreciable amounts of arsenic or antimony sulphide minerals such as orpiment, realgar, and stibnite is usually difficult or even impossible. Extractions are low and the solutions soon become ‘foul.’ The gold in such ores could be free and uncoated, and no trouble in dissolving it in clean cyanide […]
How Copper Affects Cyanidation & Leaching
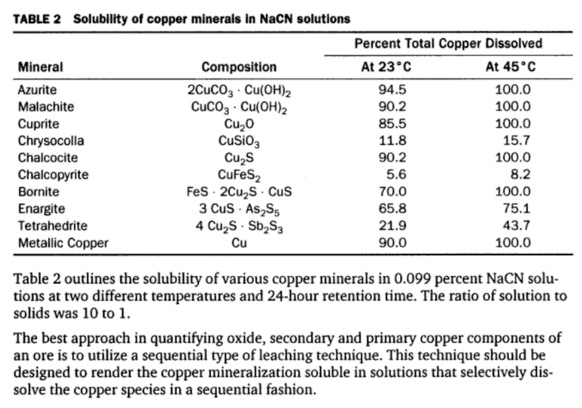
Many precious metal ores contain copper minerals in various amounts. These copper minerals dissolve in cyanide solutions to a greater or lesser degree depending on the particular copper mineral or minerals present, their fineness, and the dissolving effect of the cyanide solutions. In the process of dissolution the copper combines with, or as it is […]
