Alkali Consumption Tests
It is often desirable to ascertain by preliminary experiments the quantity of alkali which will be needed to neutralize acidity in any particular ore. In this connection a distinction is sometimes made between “ free ” and “ latent ” acidity. The free acidity is that due to substances soluble in water, and is determined […]
Organic Carbon TOC Pre-Flotation & Preg‐Robbing of Gold
Organic carbon is a common problem for base metal production and precious metal leaching. In froth flotation, liberated organic carbon is naturally hydrophobic and will be recovered with the other minerals, lowering the grade of the concentrates. It can also adsorb collectors and frothers, reducing the efficiency of the mineral recovery process. In leaching, the […]
Methods of Testing Cyanide Solutions

The method given above is difficult to apply when solutions containing soluble cyanides of zinc and other metals require to be titrated. “ A white flocculent precipitate occurs at a certain stage, probably consisting of simple (insoluble) cyanide of zinc, formed by decomposition of the soluble double cyanide: K2ZnCy4 + AgNO3 = KAgCy2 + ZnCy2 […]
Potassium Cyanide Chemistry on Minerals & Metals

The ordinary gangue of most ores (silica and silicates of the alkalies and alkaline earths) exercises no direct influence on the cyanide solution. The carbonates of the alkaline earths are also probably without influence. The decomposing effects of sulphides of the heavy metals vary with the physical state of the sulphides. Metallurgists found that dilute cyanide […]
Potassium Cyanide Leaching with Gold and Mercury
These methods are described here for convenience, as being more intelligible after the chemistry of the process has been discussed. The necessity of the presence of oxygen has already been dwelt on above. It has, however, been frequently pointed out that in the interior of a mass of ore undergoing treatment the conditions are not […]
Oxidised Pyrite Leaching by Potassium Cyanide

When the pyrites occur in tailings which have been subjected to the action of the weather for some time before treatment, compounds are formed which are more prejudicial to the solution than the sulphides. Sulphide of iron, FeS2, is oxidised by air and water, ferrous sulphate and free sulphuric acid being formed, thus: FeS2 + […]
Effect of Potassium Cyanide on Gold & Metals

The discovery that metallic gold is soluble in potassium cyanide came after studying the action of cyanides on plates of gold, and announced that they were slowly dissolved. Metallurgists pointed out that gold-leaf is dissolved by a dilute solution of the salt, and also showed that if the gold floats on the surface of the […]
Gold Vat Leaching
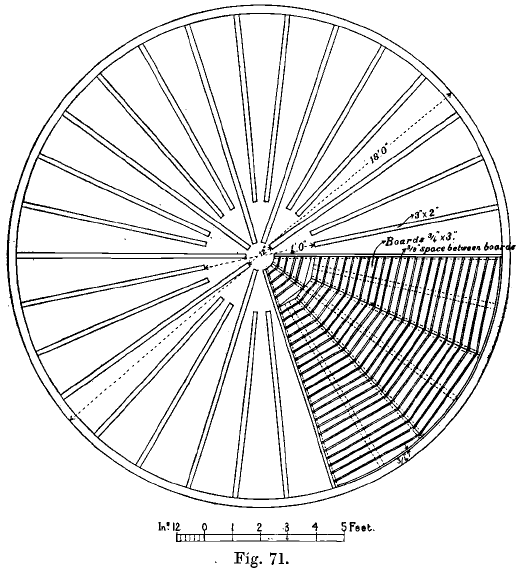
Gold Leaching done in round vats, constructed of wood, concrete, or iron and steel. If wood is used it is covered by a coating of paraffin paint, or by a mixture of asphaltum and coal-tar. Concrete and brick vats are not now advocated owing to their great cost and less convenient working, and wooden vats suffer more […]
How to Prepare and Storage of Cyanide Solution

The cyanide is usually dissolved in a little water before being added to the stock solution, as the amount of KCy present is more easily determined in a strong solution than in any other form. A special dissolving vat of small size is often provided and is placed at a higher level than the large […]
Cyanide Solution Strength Test

The strength of the cyanide solutions is tested by silver nitrate, using 10 c.c. of the strong and weak solutions respectively (diluted to about 100 c.c. with water) and taking 100 c.c. of wash-water undiluted. In each case a few drops of a 5 per cent, solution of potassium iodide are added. When the titration […]
