Table of Contents
Zircon Uses: Refractory minerals can be defined as minerals or synthetic inorganic crystal phases that have high melting points. Refractoriness has been defined by Committee C-8 of the American Society for Testing and Materials as ” the capability of maintaining the desired degree of chemical and physical identity at high temperatures and in the environment and conditions of use.” Even though the primary function of refractory minerals is dimensional and thermal stability at elevated temperatures, the definition of refractoriness implies the refractory material is expected to retain its integrity at high temperatures against a variety of destructive forces.
There are many industrial minerals such as chromite, staurolite, and olivine that have some of these characteristics, but only a few have them all. Zircon is a mineral that does contain all of the properties listed and thus is an excellent refractory material.
Zircon
Elemental zirconium is not found in nature. Instead, zirconium bonds with sodium, calcium, iron, silicon, titanium, thorium, and oxygen to form a number of different zirconium bearing minerals. Of the zirconium minerals, baddeleyite (ZrO2) and zircon (ZrSiO4) are mined on a commercial scale.
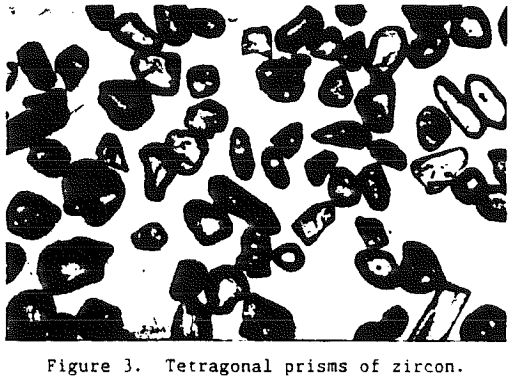
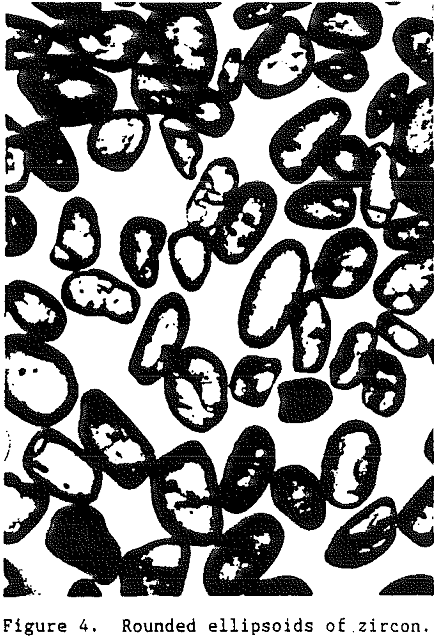
Being one of the first minerals to crystallize from an igneous melt, zircon grains are often encased as inclusions in other mineral grains such as feldspar or quartz. Thus, for a zircon grain to become a part of a commercial heavy-mineral deposit it must undergo liberation. As the rocks containing the zircon are exposed at the surface of the earth they are subjected to subaerial weathering.
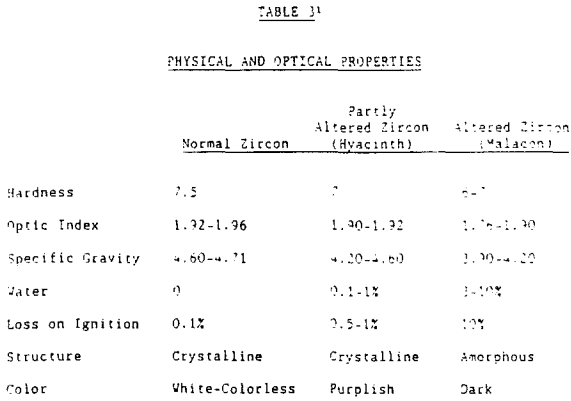
Zircon, although resistant to external alteration, is subject to internal alteration. Thorium and uranium may substitute for zirconium either in the zircon crystal lattice or in a solid solution. Initial alteration does not substantially affect such physical properties as hardness, specific gravity, or hydration. Zircon that has suffered, this initial internal alteration often has a purplish color and is referred to as hyacinth.
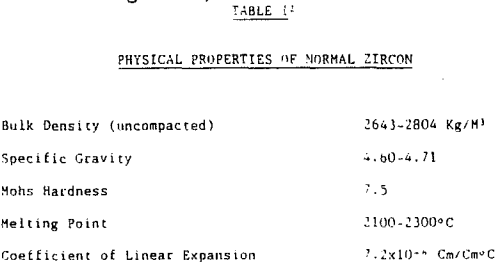
In summary, zircon is a heavy mineral (specific gravity 4.60 – 4.71) that is resistive to external destructive agents such as abrasion and chemical dissolution. It is very stable and can survive many sedimentary cycles.
Origin of Placer Deposits
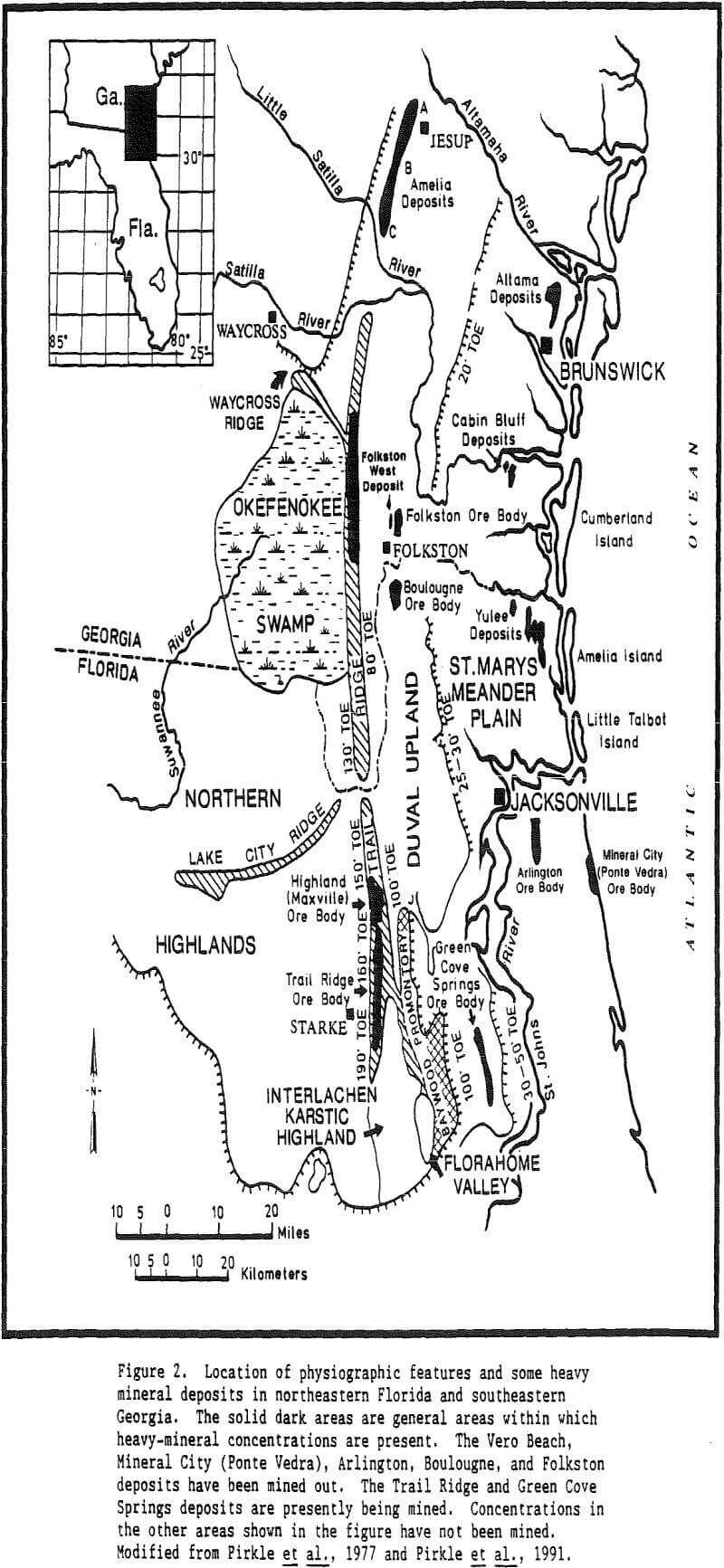
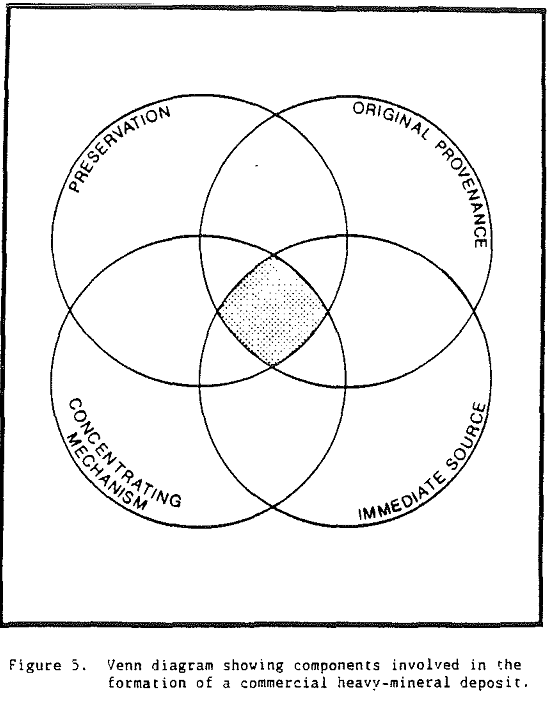
An original provenance or original source region is required for heavy minerals present in all placer deposits. However, an immediate source for heavy minerals may differ for different deposits. In the case of Trail Ridge in northern Florida, the immediate source of the sands and the heavy minerals that compose the ridge came from sands that underlie the Northern Highlands to the west of the ridge.
Zircon Properties
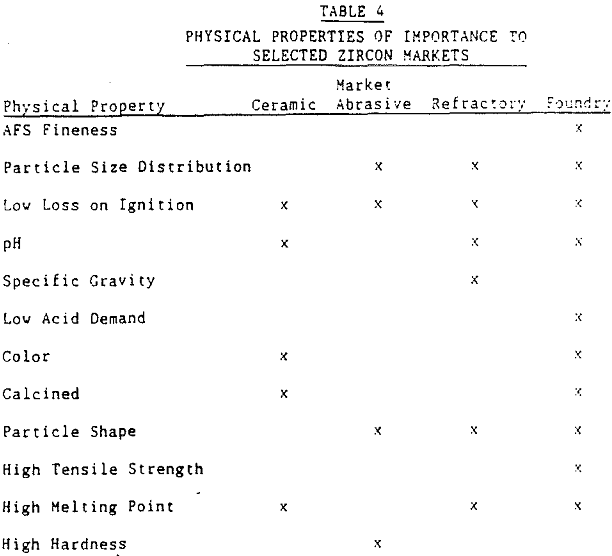
Since the refractory application of zircon products is the focus of this paper, only the physical parameters important to this industry will be discussed.
Refractory uses of zircon require a low interstitial water content. This translates into a low loss on ignition. Excessive internal radiation damage to zircon crystals (metamict zircon) can cause an increase in the loss on ignition of a zircon product. The desire of the refractory market for a low loss on ignition implies that a low picocurie/gram requirement is placed on the zircon product. To obtain this requirement requires a low uranium and thorium content of the zircon grain.
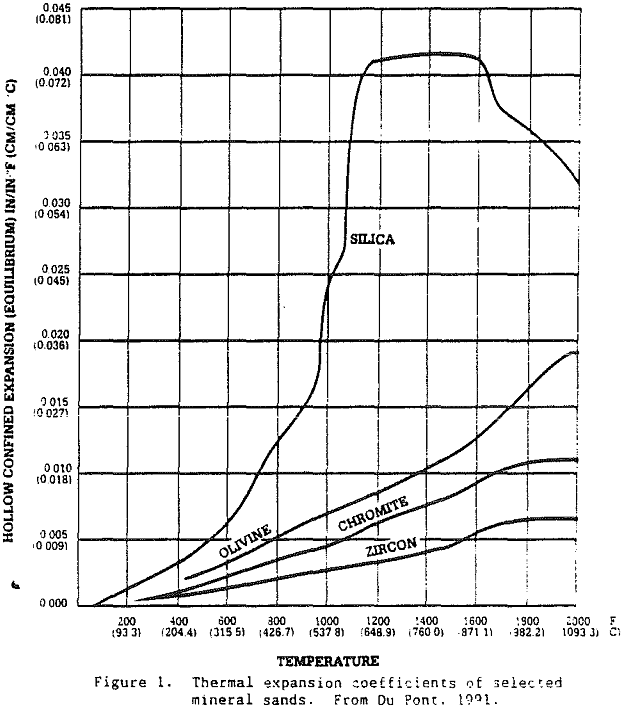
Because zircon has such a wide range of applications, there are some markets where zircon sands are under competitive pressure from less expensive silica sands, olivine sands, staurolite sands, and chromite sands. For example, Du Pont produces a staurolite refractory product from the same Trail Ridge sediments from which various grades of zircon products are produced. This product is produced for the nonferrous segment of the foundry market. In casting metals such as brass and aluminum, the staurolite product offers a relatively high melting point, good refractoriness, and low thermal expansion. Thus, zircon sand producers provide a variety of mineral products.