Table of Contents
Cadmium is not the principal recoverable constituent in any ore, but it does occur at a ratio of about 1:200 with zinc. If lead and zinc are considered major products, then the major metal byproduct of the zinc industry was the more than 3 million pounds of cadmium produced in the United States in 1978 (15). Although cadmium is a valuable commodity, much of it is lost during zinc processing; in addition, the U.S. Environmental Protection Agency has identified cadmium as a toxic pollutant. Therefore, it is important to understand the behavior of cadmium during zinc concentrate processing so as to recover this valuable metal and to contain it as a possible health hazard. In keeping with its goals to minimize the undesirable environmental conflicts and occupational hazards associated with metal processing operations, and to maximize the productivity of mineral processing, the Bureau has conducted research to determine the behavior of cadmium during roasting of zinc concentrates. The investigators chose roasting for this study because it is common to both the hydrometallurgy and pyrometallurgy processing of zinc and is the first processing stage in which cadmium separation appears possible.
The roasting of zinc sulfide concentrates has been the subject of much research, as have the oxidation of sphalerite, the most important zinc sulfide mineral, and the formation of zinc ferrite, ZnFe2O4. However, less attention has been paid to the behavior of cadmium during roasting, even though considerable loss of this valuable byproduct occurs at this step in the zinc-smelting process. In an earlier Bureau report, Kenworthy and Absalom showed that cadmium, as well as germanium and lead, could be volatilized from zinc concentrate by heating at temperatures ranging from 700° to 1,050° C in an inert or reducing atmosphere. Their samples contained a low concentration of iron, and their assumed cadmium sulfide and cadmium oxide vapor pressures were not in accord with more recent data. Kellogg has since shown that the vaporization of cadmium should be much greater than previously indicated, if both the oxide and sulfide were present.
Additional data on the disposition of cadmium during zinc sulfide roasting were recently presented in three reports. In the first of these, Roggero reported increased cadmium removal after using temperatures higher than the usual roasting temperature of 950° C, and also noted the formation of an iron layer on the concentrate particles. Triplett analyzed cadmium paths in a commercial smelter, confirmed a poor cadmium recovery, and indicated that improvements could be made if more attention were paid to cadmium recovery during roasting. Finally, Stoilzova pointed out that cadmium retained in roasted zinc concentrate was associated with iron and incompletely roasted sulfides. The present study shows why iron is significant in preventing the volatilization of cadmium during roasting.
Research and Discussion
Materials
This investigation began with the selection of several commercial zinc concentrates and samples of materials from accessible points in commercial roasters. Because microscopic examination of the concentrates showed no major differences in sources, only five concentrates were selected for closer examination and laboratory experimentation. Analyses of these concentrates are given in tables 1 and 2. In commercial practice, concentrates 1 and 2 are first heated to over 950° C in an oxygen-lean atmosphere (preroasted), then roasted in air, at approximately 950° C, and finally sintered before cooling. The other three concentrates were not preroasted. Both the preheating and roasting steps are of interest to this study.
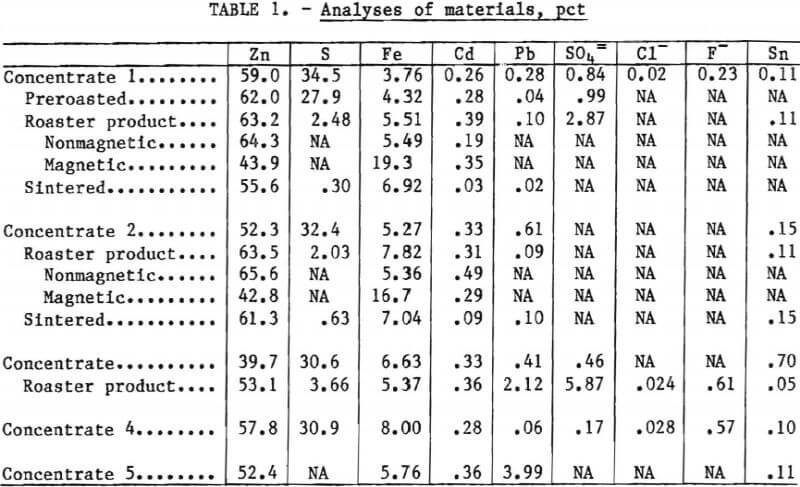
Oxidation Kinetics
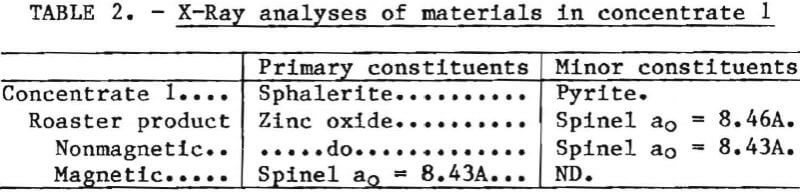
The kinetics of air-roasting zinc sulfide concentrate were studied by continuously weighing a concentrate sample as it was exposed to a stream of air at a constant temperature. Typical weight-loss curves representing the oxidation of metal sulfides in a concentrate sample are shown in figure 1. The weight changes are caused primarily by the loss of sulfur as sulfur dioxide and the formation of metal oxides. These curves show two reaction regimes representing an initial, fast reaction and a second, slower reaction. Differential thermal analyses (DTA) helped identify the two reactions.
A DTA scan of a concentrate sample in air is shown in figure 2. The first exothermic peak, occurring at about 500° C, corresponded to oxidation with pyrite and the high-iron components of the concentrate. The larger peak, occurring at about 700° C, corresponded to the single peak obtained with sphalerite or reagent-grade zinc sulfide under similar conditions. In the apparatus used in this investigation, air oxidation of reagent-grade cadmium sulfide produced an exothermic DTA peak at slightly over 700° C, with a minor side peak at 700° C. A prepared solid solution of 80 pct zinc sulfide, 10 pct cadmium sulfide, and 10 pct iron sulfide gave a single DTA peak generally corresponding to the zinc sulfide peak.
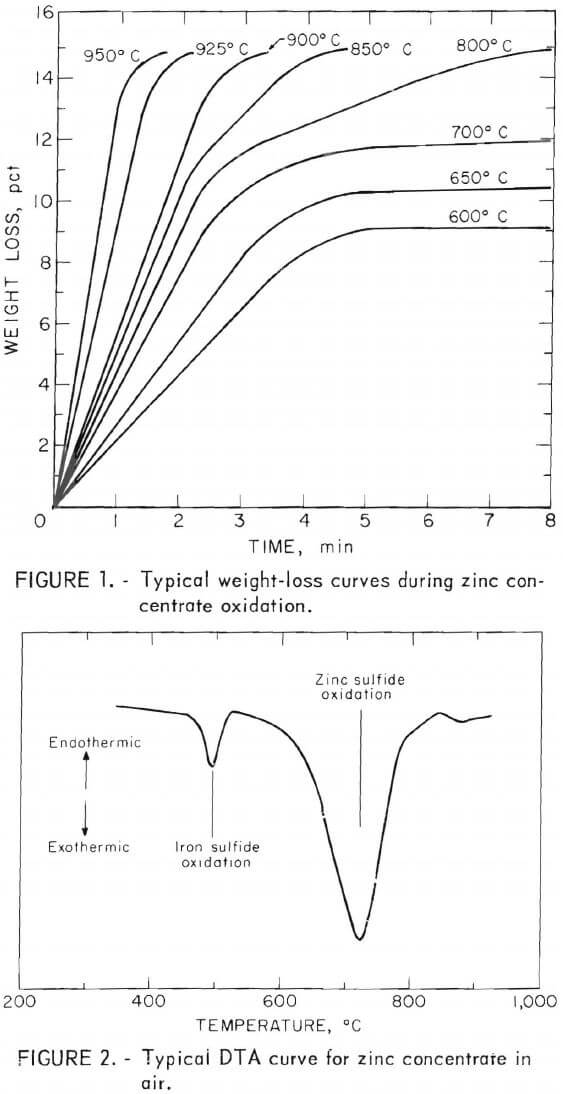 These findings can be interpreted as an oxidation of zinc concentrate in which separate particles containing primarily iron sulfide are oxidized first, with a slower and simultaneous oxidation of the predominantly zinc particles. However, close examination of the rapid reaction portion of the weight loss curves showed a greater weight loss than was expected on the basis of the iron content of the concentrate alone. This inconsistency was resolved when the oxidation of a concentrate sample was observed under a microscope. At temperatures above 600 C, exothermic oxidation of high-iron particles occurred when air was allowed to flow over the sample. This temperature increase was evidenced by an increased glowing that lasted for only a short time. To determine which particles glowed, a magnet was used to separate some particles with higher iron content from the bulk of the concentrate. During oxidation, high-iron particles alongside the remaining concentrate were observed through a microscope. Those particles with high-iron content were observed to glow. Therefore, the higher than expected weight change in these laboratory experiments, in which excess oxygen was always available, could be explained by local heating and hence faster oxidation of sulfides adjacent to the fast-reacting, high-iron particles. Later in this report, the behavior of the particles will be contrasted with the oxidation of particles in a deep bed where excess oxygen is not always available.
These findings can be interpreted as an oxidation of zinc concentrate in which separate particles containing primarily iron sulfide are oxidized first, with a slower and simultaneous oxidation of the predominantly zinc particles. However, close examination of the rapid reaction portion of the weight loss curves showed a greater weight loss than was expected on the basis of the iron content of the concentrate alone. This inconsistency was resolved when the oxidation of a concentrate sample was observed under a microscope. At temperatures above 600 C, exothermic oxidation of high-iron particles occurred when air was allowed to flow over the sample. This temperature increase was evidenced by an increased glowing that lasted for only a short time. To determine which particles glowed, a magnet was used to separate some particles with higher iron content from the bulk of the concentrate. During oxidation, high-iron particles alongside the remaining concentrate were observed through a microscope. Those particles with high-iron content were observed to glow. Therefore, the higher than expected weight change in these laboratory experiments, in which excess oxygen was always available, could be explained by local heating and hence faster oxidation of sulfides adjacent to the fast-reacting, high-iron particles. Later in this report, the behavior of the particles will be contrasted with the oxidation of particles in a deep bed where excess oxygen is not always available.
The attempt to obtain quantitative data for the fast oxidation of the high-iron fraction of zinc concentrate was hampered above 600° C by the self-heating of the concentrate. Below 600° C, the reaction is of little importance in roasting. Reaction rate data for the high-zinc fraction were obtained by using small, 50-milligram samples that rested on quartz wool in an open-bottomed quartz bucket. Even at high reaction rates, with this configuration, oxygen diffusion to the sulfide surfaces was not rate limiting in rapidly flowing air. The resulting data are presented in figure 3. This straight-line Arrhenius relationship indicates that one reaction mechanism prevails over this extended temperature range. Similarity in oxidation rates of the various concentrates and the Arrhenius (apparent) activation energy of 50 kcal, agree well with results obtained by other investigators.
Particle size was determined to be a factor in the oxidation of the concentrates. As expected, finer particles, which were ground from the same materials to obtain a greater surface area per unit weight of sample, showed a higher reaction rate measured as a weight-percent change. Experiments giving high apparent reaction rates also showed a greater net weight loss at the break in the weight-loss curve (fig. 1) between the fast and slower reactions. This is due to the rate of heat loss because heat from a very rapid initial reaction is more effective in heating up the surrounding sample, causing greater reactivity than the same amount of heat generated over a longer time span.
Distribution of Elements
Sulfide minerals are generally a mixture of solid solutions, rather than pure distinct species, and reactions involving sulfide minerals and the resulting products are not accurately described by conventional chemical equations. Therefore, it was useful to examine the elemental distribution in the solid reactants and products as a method of describing the chemistry of sulfide roasting.
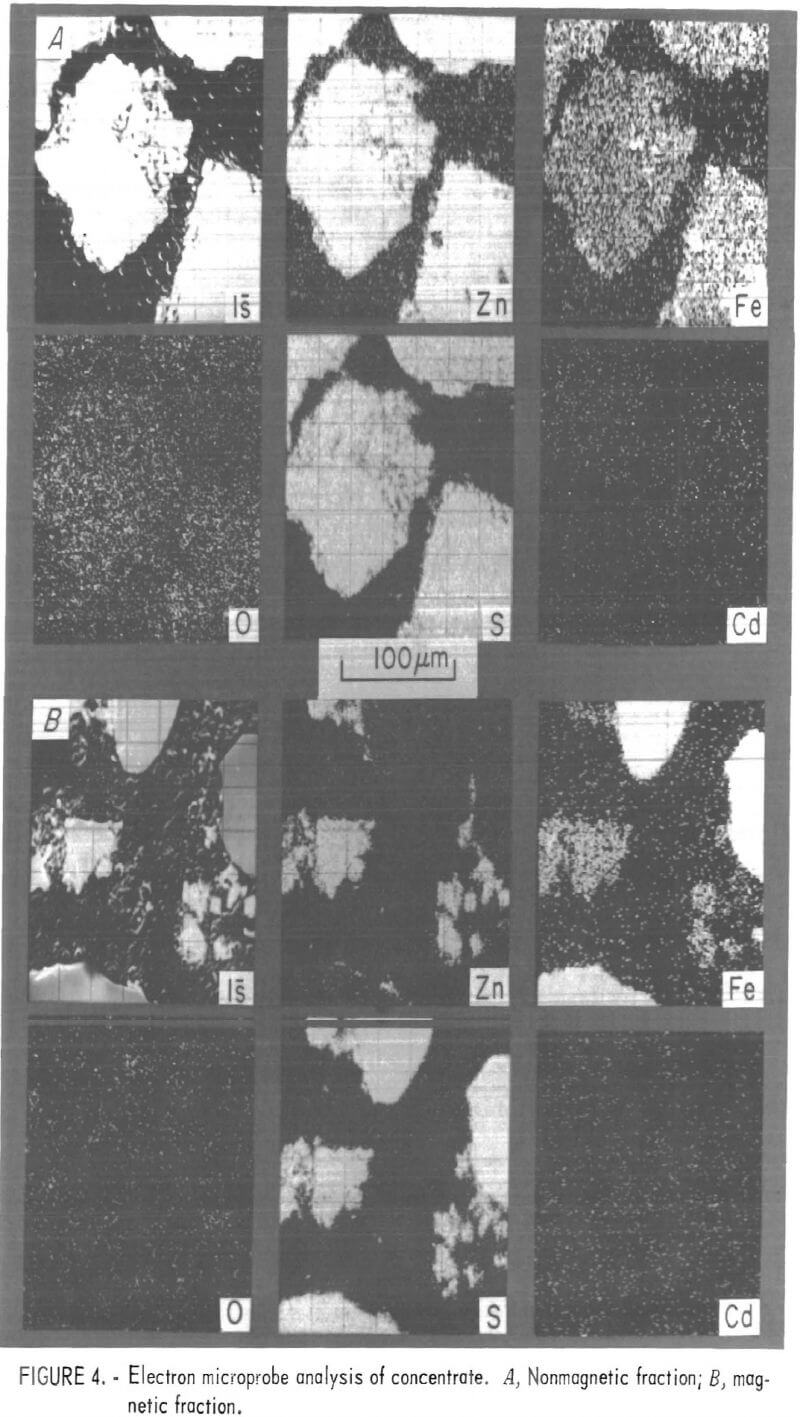
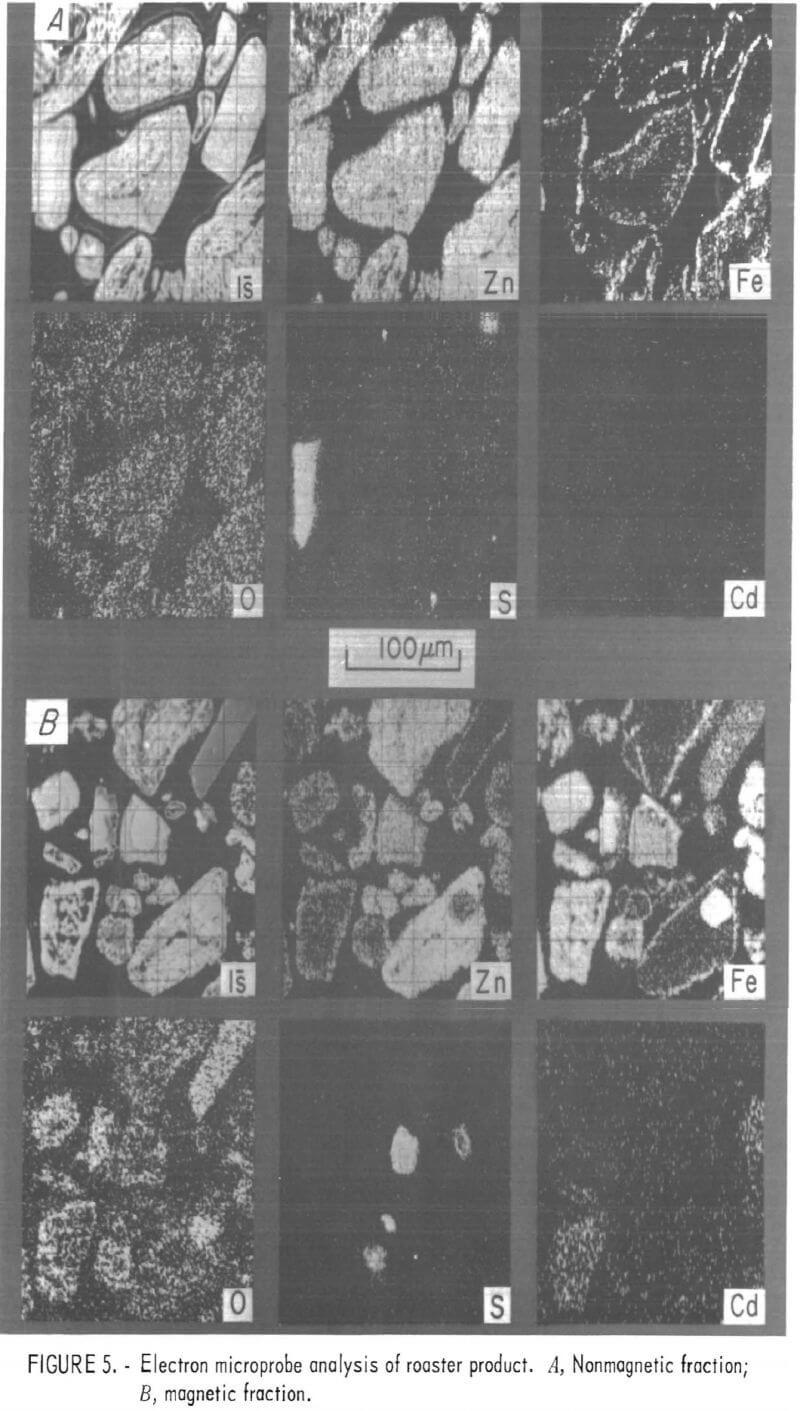
Distributions of zinc, sulfur, iron, cadmium, and oxygen were examined in roaster feed, roaster product, and prepared sulfide samples using an electron microprobe. The results are shown in the electron probe micrographs, figures 4 through 7. The samples were prepared for these electron microprobe examinations by mounting a layer of particles in plastic and then polishing the particles to approximately one-half the particle diameter. In these figures, each lettered series shows the same field of view of sample particles. Each electronprobe micrograph labeled is was made by using the sample current of the microprobe and closely resembles the sample as seen with an optical microscope. Each of the other electronprobe micrographs in the series shows the distribution of a particular element. These elemental distributions are qualitative, and the intensities are not necessarily a good indication of relative concentration.
Under preliminary examination, the electronprobe micrographs of both the roaster feeds and roaster products showed two types of particles. In both the feed and product samples, the particles could be conveniently separated as magnetic and nonmagnetic fractions, as shown in figures 4 and 5. A small handheld magnet was used to separate approximately 5 pct of roaster feed and roaster product as the magnetic fractions.
Nonmagnetic roaster feed particles appear to be primarily zinc sulfide, with an iron sulfide evenly distributed throughout (fig. 4A). The magnetic fraction (fig. 4B) shows particles consisting essentially of iron sulfide, with finer particles of the nonmagnetic material. These observations are reinforced by the analyses listed in tables 1 and 2.
Roaster products are more complex, as shown in figure 5. After roasting, both magnetic and nonmagnetic fractions appear to be somewhat laminar with iron-rich outer layers surrounding predominately zinc particles. In addition, the predominately iron particles in the magnetic fraction show a zinc-rich outer layer. Oxygen replaces sulfur in all but a few particles, which retain unoxidized sulfide cores. And finally, cadmium was barely evident in the electronprobe micrographs of a few iron-rich particles in the magnetic fraction.
Because the low concentration of cadmium in the roaster feed and product did not allow adequate observation of this element, and in order to investigate the cadmium-iron relationship, two special sulfide samples were prepared. One sample contained zinc and cadmium sulfides; the other contained zinc, iron, and cadmium sulfides. Both were prepared by pressing mixtures of the appropriate reagent-grade sulfides into pellets. Heating these pellets in

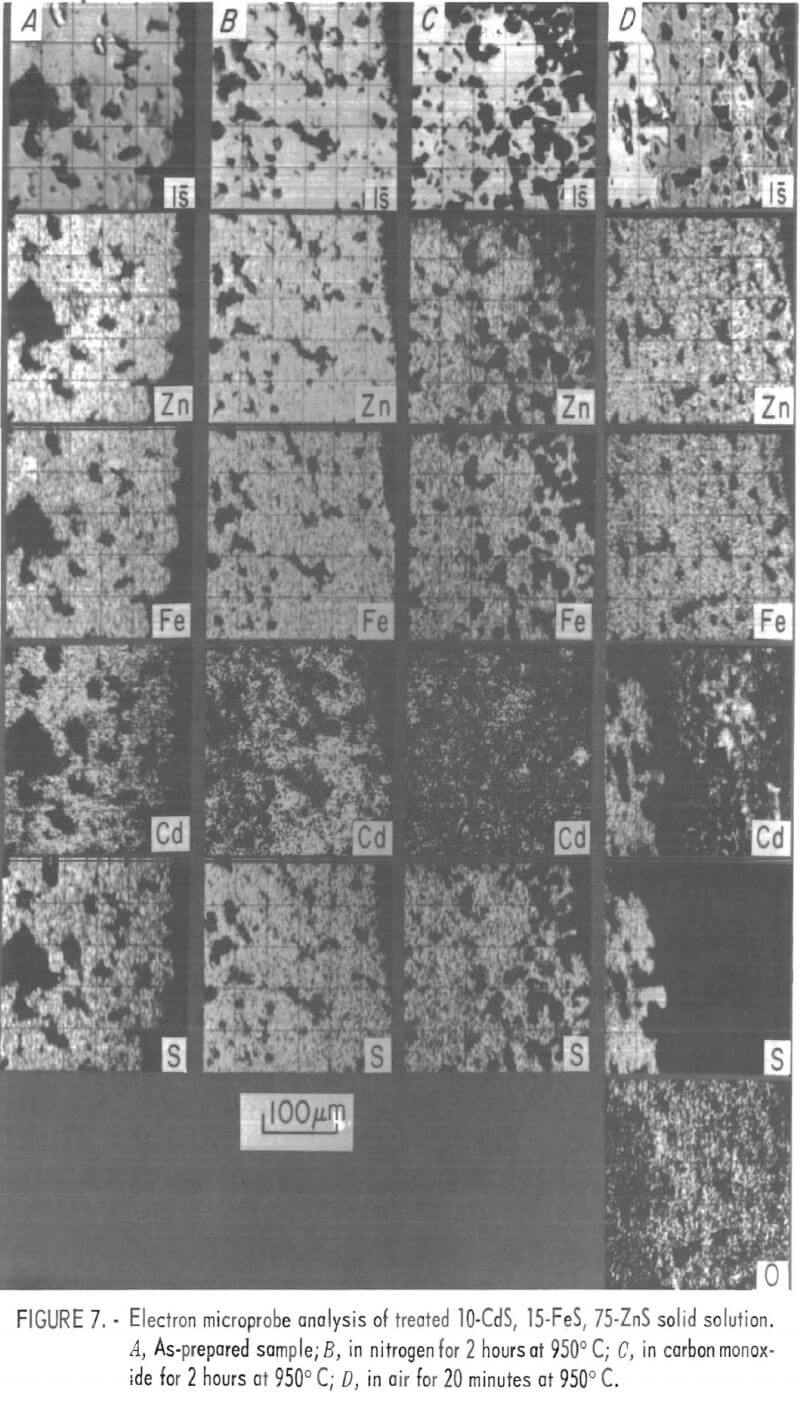
evacuated glass ampules for several hours at 950° C gave a homogeneous material that was ground into finer particles for testing. The homogeneity of the resulting materials is demonstrated in the first columns (A) of figures 6 and 7.
Small samples of each material were heated in either flowing air, nitrogen, or carbon monoxide, while being continuously weighed until the sample weight was constant. Fresh samples were similarly heated, but only until one-half of the previously noted weight change was attained. These half-reacted samples were cooled quickly while still under the same atmosphere. The remaining columns in figure 6 and 7 show the elemental distributions in cross sections of the half-reacted particles. Because the zinc-cadmium particles were smaller, several particles are shown in each sample; the zinc-iron cadmium particles were larger, porous, and only the edge of one particle is shown in each electronprobe micrograph. However, these differences do not affect the following discussion.
In the particles with homogeneous distributions of zinc, iron, and cadmium in a sulfide matrix, only cadmium changed when heated in nitrogen or carbon monoxide. Similar observations were made by Schlechten. Under a neutral or reducing atmosphere, the cadmium appears to be depleted at and near the solid surface, leaving a concentration gradient indicative of a diffusion-controlled mechanism. Depletion of cadmium seems to occur more rapidly in a carbon monoxide atmosphere.
When an atmosphere of air was used to partially oxidize sulfide particles at high temperatures, a sharp demarcation was observed between the unreacted sulfide and the oxidized portion (figs. 6C and 7D). The presence of iron under these conditions greatly affected the behavior of cadmium. In the particles with zinc and cadmium but without iron, cadmium was observed in the unreacted sulfide but not in the oxide layer. With iron present, cadmium was observed both in the sulfide layer and (in a somewhat rearranged form) in the oxide layer. A distinctive feature of this latter condition was a cadmium- free area in the oxide layer adjacent to and surrounding the unreacted sulfide. It will be shown that the vapor pressure of cadmium is highest in this area.
Thermodynamic Considerations
Roasting is a dynamic process involving gas flow, heating, vaporization, and chemical reaction between gaseous and solid phases. As has been shown, the solid phase is not homogeneous, but an intimate mixture. The gas phase, because of rapid chemical reactions, may have large composition gradients. In spite of these departures from equilibrium, thermodynamics can be useful in elucidating the behavior of cadmium during roasting.
Sulfur dioxide and oxygen are the important reactive gases present in roasting. Predominance diagrams plotted in reference to the pressures of these gases are given in figures 8 through 10 for iron, cadmium, and zinc, respectively. Data for these diagrams were taken from references. The only temperature considered here is 927° C (1,200 K) because it is in
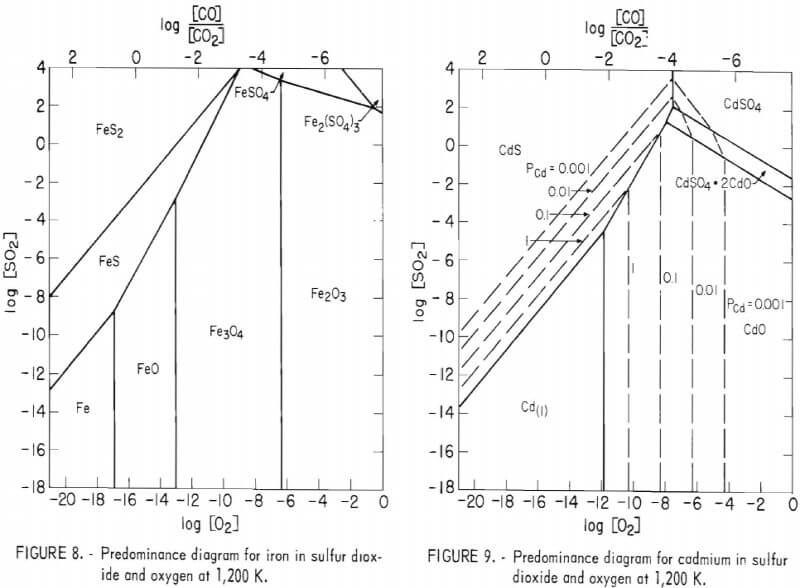
the range of most commercial roasting temperatures, and the predominance diagrams remain relatively unchanged at lower temperatures. Also, reaction rates are low and of less importance at lower temperatures.
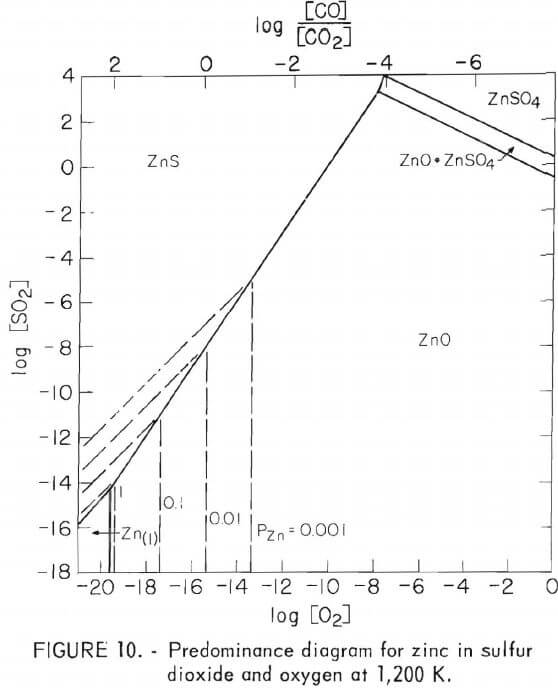 The predominance diagrams show that the lines separating sulfide and oxide phases vary for the three metals. At a given sulfur dioxide pressure, iron oxides can exist in equilibrium with iron sulfide at an oxygen pressure nearly one order of magnitude lower than a similar relationship for the zinc compounds, and two orders of magnitude lower than this relationship for the cadmium compounds. This means once iron sulfide begins to react with available oxygen, the local oxygen pressure could be lowered sufficiently within small regions to prevent oxidation of nearby zinc and cadmium sulfides, even while the iron sulfide continues to react. By similar reasoning, the oxidation of magnetite to hematite would not occur if zinc and cadmium sulfides were present.
The predominance diagrams show that the lines separating sulfide and oxide phases vary for the three metals. At a given sulfur dioxide pressure, iron oxides can exist in equilibrium with iron sulfide at an oxygen pressure nearly one order of magnitude lower than a similar relationship for the zinc compounds, and two orders of magnitude lower than this relationship for the cadmium compounds. This means once iron sulfide begins to react with available oxygen, the local oxygen pressure could be lowered sufficiently within small regions to prevent oxidation of nearby zinc and cadmium sulfides, even while the iron sulfide continues to react. By similar reasoning, the oxidation of magnetite to hematite would not occur if zinc and cadmium sulfides were present.
The predominance diagrams also show the relative volatilities of the metals. The dashed vertical lines in figures 9 and 10 represent equilibrium vapor pressures of the metals above 0.001 atmosphere. Iron volatility is nil, and zinc exhibits only negligible vapor pressure even at very low oxygen and sulfur dioxide pressures. However, cadmium vapor pressure is over four orders of magnitude greater, rising to approximately one-third of an atmosphere under conditions found possible on a microscopic scale in roasting. These higher pressures occur on the equilibrium line between cadmium oxide and sulfide, indicating that during oxidative roasting, cadmium would be most likely to escape by vaporization at the interface between the still unreacted sulfide and the advancing oxide product. Figure 7D shows a cadmium free zone at this interface, and also indicates that the cadmium is trapped away from the interface and in the oxide product where the oxygen pressure would be greater.
The nature of the trapped cadmium is not known and was not discernible by X-ray diffraction. A spinel pattern was found that had a lattice parameter between Fe2O3 and Fe2ZnO4, indicating FeOxZnOx-1 Fe2O3 and could include Fe2CdO4. On the other hand, cadmium could also be in the form of cadmium oxide and be sufficiently dispersed so as to be undetected by X-ray diffraction. It would be helpful to know the thermodynamic properties of Fe2CdO4, but these data are not available. However, during the course of this work, ancillary experiments were conducted that indicated the relative stabilities of the spinels, and, consequently, suggested a mechanism for cadmium retention. First, when zinc vapor was passed over Fe2O3 powder at 900° C, the zinc was rapidly and evenly incorporated into the oxide particles as shown by electron microprobe examination. If Fe3O4 particles were used instead of Fe2O3 the zinc was found only at or near the particle surfaces. Second, when zinc vapors were passed over FeCdO4 particles at 900° C, the zinc replaced cadmium, but if cadmium vapors were passed over FeZnO4, no change was observed.
From these observations and the predominance diagrams, one can conclude that cadmium will be much more volatile than zinc at some point during roasting. Thus, although there is much more zinc present, and despite the fact that the zinc spinel is the more stable, the much greater volatility of the cadmium allows it to readily come in contact with any available iron oxide and be converted to a nonvolatile oxide. This trapping of cadmium would be further enhanced if the iron layer observed on the roaster product is able to form before the cadmium vaporizes from the oxidizing sulfide particles.
Finally, the [CO]/[CO2] equilibrium ratios, shown at the top of the pre-dominance diagrams, demonstrate that Fe2O3, and perhaps the spinels, can be prevented from forming by applying a relatively low partial pressure of carbon monoxide. Such reducing conditions were shown to be effective in removing most of the cadmium during a preroast treatment at 950° C. A preroast volatilization of cadmium is being investigated.
Conclusions
- Oxidation of zinc sulfide concentrate is rapid at roasting temperatures, with the reaction being limited by gaseous diffusion to the interface.
- The high-iron fraction of the concentrate reacts most rapidly.
- An iron-containing layer forms during roasting on the particles that were originally primarily zinc sulfide.
- The distribution of cadmium in zinc concentrate is not known, but cadmium is more associated with iron after roasting.
- Cadmium will volatilize from concentrate to some extent in an inert atmosphere, but more readily in carbon monoxide.
- Cadmium will also volatilize from iron-free cadmium sulfide-zinc sulfide solid solution during oxidation, but is trapped in the oxide product if iron is present.
It appears that oxidized iron is the major factor preventing cadmium from volatilizing during roasting. Two factors enhance the effectiveness of iron in this process. The first is if the iron oxide is the first oxide to form: the second is if an iron layer forms around individual concentrate particles during roasting. The role of the iron may be to catalyze the oxidation of cadmium vapor, to form the nonvolatile cadmium spinel, or a combination of both. A nonoxidizing or reducing preroast avoids the effect of oxidized iron, and it increases the removal of cadmium prior to roasting.
The Bureau of Mines has undertaken research to elucidate the chemistry involved in the efficient recovery and control of minor elements in base metal smelting operations. These elements should be effectively recovered not only because they have commercial value, but also because of their potential hazard to workers health and the environment, if they are not controlled during processing. Cadmium, a minor metal in zinc ores, is the subject of this report.
The roasting step of the complex zinc-smelting process was chosen for investigation because of the high potential for removing cadmium by volatilization during this primary stage. Commercial roaster feed materials were used to determine the sequence of reactions and the effect of these reactions on the volatilization of cadmium. Electron microprobe examination of both the commercial roaster product and oxidized sulfide solid solutions prepared in the laboratory showed that oxidized iron was of prime importance in preventing cadmium volatilization. The mechanism by which iron prevents cadmium volatilization is proposed, and a preheating technique is recommended for more effectively recovering cadmium prior to roasting.
